Abstract
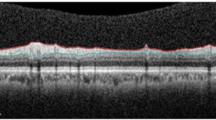
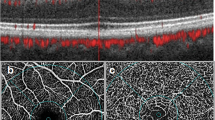
Previous reports of ocular abnormalities in Huntington’s disease (HD) have detailed eye movement disorders. The objective of this case–control study was to investigate optic nerve and macular morphology in HD using optical coherence tomography (OCT). A total of 26 HD patients and 29 controls underwent a thorough ophthalmic examination including spectral domain OCT scans of the macula and peripapillary retinal nerve fibre layer (RNFL). Genetic testing results, disease duration, HD disease burden scores and Unified HD Rating Scale motor scores were acquired for HD patients. Temporal RNFL thickness was significantly reduced in the HD group (62.3 vs. 69.8 μm, p = 0.005), and there was a significant negative correlation between temporal RNFL thickness and disease duration (R 2 = −0.51, p = 0.04). Average peripapillary RNFL thickness was not significantly different between the HD and control groups. There was a significant negative correlation between macular volume and disease duration (R 2 = −0.71, p = 0.002), and motor scores (R 2 = −0.56, p = 0.01). Colour vision was significantly poorer in the HD group. Temporal RNFL is preferentially thinned in HD patients, possibly implicating mitochondrial dysfunction as the temporal RNFL is reduced in the patients with some mitochondrial disorders, including Leber’s hereditary optic neuropathy. The correlation between the decrease in macular volume and temporal RNFL, and increasing disease severity suggests that OCT may be a useful biomarker for disease progression in HD. Larger, longitudinal studies are required.






Similar content being viewed by others
References
Purdon SE, Mohr E, Ilivitsky V, Jones BD (1994) Huntington’s disease: pathogenesis, diagnosis and treatment. J Psychiatry Neurosci 19(5):359–367
Martin JB, Gusella JF (1986) Huntington’s disease. Pathogenesis and management. N Engl J Med 315(20):1267–1276
The Huntington’s Disease Collaborative Research Group and The Hungtinton Study Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s disease collaborative research group. Cell 72(6):971–83
Stout JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC et al (2011) Neurocognitive signs in prodromal Huntington disease. Neuropsychology 25(1):1–14
Ha AD, Fung VSC (2012) Huntington’s disease. Curr Opin Neurol 25(4):491–498
Blekher T, Johnson SA, Marshall J, White K, Hui S, Weaver M et al (2006) Saccades in presymptomatic and early stages of Huntington disease. Neurology 67(3):394–399
Peltsch A, Hoffman A, Armstrong I, Pari G, Munoz DP (2008) Saccadic impairments in Huntington’s disease. Exp Brain Res 186(3):457–469
Lasker AG, Zee DS, Hain TC, Folstein SE, Singer HS (1988) Saccades in Huntington’s disease: slowing and dysmetria. Neurology 38(3):427–431
Leigh RJ, Newman SA, Folstein SE, Lasker AG, Jensen BA (1983) Abnormal ocular motor control in Huntington’s disease. Neurology 33(10):1268–1275
Anderson TJ, MacAskill MR (2013) Eye movements in patients with neurodegenerative disorders. Nat Rev Neurol 9(2):74–85
Sakai RE, Feller DJ, Galetta KM, Galetta SL, Balcer LJ (2011) Vision in multiple sclerosis: the story, structure-function correlations, and models for neuroprotection. J Neuroophthalmol 31(4):362–373
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W et al (1991) Optical coherence tomography. Science 254(5035):1178–1181
Wojtkowski M, Srinivasan VJ, Ko TH, Fujimoto JG, Kowalczyk A, Duker JS (2004) Ultrahigh-resolution, high-speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Opt Express 12(11):2404–2422
Altintas O, Iseri P, Ozkan B, Caglar Y (2008) Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Doc Ophthalmol 116(2):137–146
Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML et al (2006) Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 113(2):324–332
Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ et al (2007) Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 69(16):1603–1609
Greenberg BM, Frohman E (2010) Optical coherence tomography as a potential readout in clinical trials. Ther Adv Neurol Disord 3(3):153–160
Iseri PK, Altinas O, Tokay T, Yuksel N (2006) Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol 26(1):18–24
Klistorner A, Garrick R, Barnett MH, Graham SL, Arvind H, Sriram P et al (2013) Axonal loss in nonoptic neuritis eyes of patients with multiple sclerosis linked to delayed visual evoked potential. Neurology 80(3):242–245
Lange AP, Sadjadi R, Zhu F, Alkabie S, Costello F, Traboulsee AL (2013) Spectral-domain optical coherence tomography of retinal nerve fiber layer thickness in NMO patients. J Neuroophthalmol 33(3):213–219
Llufriu S, Sepulveda M, Blanco Y, Marin P, Moreno B, Berenguer J et al (2014) Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. Plos One 9(12):e113936
Merle H, Olindo S, Donnio A, Richer R, Smadja D, Cabre P (2008) Retinal peripapillary nerve fiber layer thickness in neuromyelitis optica. Invest Ophthalmol Vis Sci 49(10):4412–4417
Polo V, Garcia-Martin E, Bambo MP, Pinilla J, Larrosa JM, Satue M et al (2014) Reliability and validity of Cirrus and Spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer’s disease. Eye (Basingstoke) 28(6):680–690
Satue M, Garcia-Martin E, Fuertes I, Otin S, Alarcia R, Herrero R et al (2013) Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson’s disease patients. Eye 27(4):507–514
Galetta KM, Calabresi PA, Frohman EM, Balcer LJ (2011) Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics 8(1):117–132
Reilmann R, Bohlen S, Kirsten F, Ringelstein EB, Lange HW (2011) Assessment of involuntary choreatic movements in Huntington’s disease–toward objective and quantitative measures. Mov Disord 26(12):2267–2273
Penney JB Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH (1997) CAG repeat number governs the development rate of pathology in Huntington’s disease. Ann Neurol 41(5):689–692
Kersten HM, Roxburgh RH, Danesh-Meyer HV (2014) Ophthalmic manifestations of inherited neurodegenerative disorders. Nat Rev Neurol 10(6):349–362
Fortuna F, Barboni P, Liguori R, Valentino ML, Savini G, Gellera C et al (2009) Visual system involvement in patients with Friedreich’s ataxia. Brain 132(Pt 1):116–123
Sitarz KS, Chinnery PF, Yu-Wai-Man P (2012) Disorders of the optic nerve in mitochondrial cytopathies: new ideas on pathogenesis and therapeutic targets. Curr Neurol Neurosci Rep 12(3):308–317
Barboni P, Savini G, Valentino ML, Montagna P, Cortelli P, De Negri AM et al (2005) Retinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathy. Ophthalmology 112(1):120–126
Klebe S, Depienne C, Gerber S, Challe G, Anheim M, Charles P et al (2012) Spastic paraplegia gene 7 in patients with spasticity and/or optic neuropathy. Brain 135(Pt 10):2980–2993
Li J (2012) Inherited neuropathies. Semin Neurol 32(3):204–214
Sadun AA, Win PH, Ross-Cisneros FN, Walker SO, Carelli V (2000) Leber’s hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc 98:223–232
Zuccato C, Valenza M, Cattaneo E (2010) Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol Rev 90(3):905–981
Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA et al (2011) Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: implications for selective neuronal damage. Hum Mol Genet 20(7):1438–1455
Orr AL, Li S, Wang CE, Li H, Wang J, Rong J et al (2008) N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci 28(11):2783–2792
Fraser JA, Biousse V, Newman NJ (2010) The neuro-ophthalmology of mitochondrial disease. Surv Ophthalmol 55(4):299–334
Helmlinger D, Yvert G, Picaud S, Merienne K, Sahel J, Mandel J-L et al (2002) Progressive retinal degeneration and dysfunction in R6 Huntington’s disease mice. Hum Mol Genet 11(26):3351–3359
Paulus W, Schwarz G, Werner A, Lange H, Bayer A, Hofschuster M et al (1993) Impairment of retinal increment thresholds in Huntington’s disease. Ann Neurol 34(4):574–578
Petrasch-Parwez E, Saft C, Schlichting A, Andrich J, Napirei M, Arning L et al (2005) Is the retina affected in Huntington disease? Acta Neuropathol 110(5):523–525
Petrasch-Parwez E, Habbes H-W, Weickert S, Lobbecke-Schumacher M, Striedinger K, Wieczorek S et al (2004) Fine-structural analysis and connexin expression in the retina of a transgenic model of Huntington’s disease. J Comp Neurol 479(2):181–197
Batcha AH, Greferath U, Jobling AI, Vessey KA, Ward MM, Nithianantharajah J et al (2012) Retinal dysfunction, photoreceptor protein dysregulation and neuronal remodelling in the R6/1 mouse model of Huntington’s disease. Neurobiol Dis 45(3):887–896
Li M, Yasumura D, Ma AAK, Matthes MT, Yang H, Nielson G et al (2013) Intravitreal administration of HA-1077, a ROCK inhibitor, improves retinal function in a mouse model of huntington disease. Plos One 8(2):e56026
Ragauskas S, Leinonen H, Puranen J, Ronkko S, Nymark S, Gurevicius K et al (2014) Early retinal function deficit without prominent morphological changes in the R6/2 mouse model of Huntington’s disease. Plos One 9(12):e113317
Mizuno K, Asaoka M (1976) Cycloscopy and fluorescein cycloscopy. Investig Ophthalmol 15(7):561–564
Dorsey ER, Beck CA, Darwin K, Nichols P, Brocht AFD, Biglan KM et al (2013) Natural history of Huntington disease. JAMA Neurol 70(12):1520–1530
The Huntington’s Disease Collaborative Research Group and The Hungtinton Study Group (1996) Unified Huntington’s disease rating scale: reliability and consistency. Huntington Study Group. Mov Disord 11(2):136–42
Stroet A, Linker RA, Gold R (2013) Advancing therapeutic options in multiple sclerosis with neuroprotective properties. J Neural Transm 120(Suppl 1):S49–S53
Acknowledgments
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kersten, H.M., Danesh-Meyer, H.V., Kilfoyle, D.H. et al. Optical coherence tomography findings in Huntington’s disease: a potential biomarker of disease progression. J Neurol 262, 2457–2465 (2015). https://doi.org/10.1007/s00415-015-7869-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7869-2