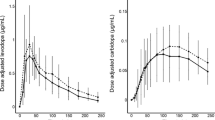
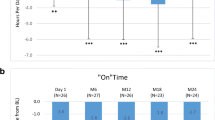
Abstract
Background
Chronic levodopa treatment in Parkinson’s disease (PD) may promote undesirable motor and non-motor fluctuations. Compared to chronic oral levodopa treatment, continuous infusion of levodopa/carbidopa intestinal gel (LCIG) in advanced PD reduces motor fluctuations. However, differences in their effect on acute non-motor changes were not formally demonstrated.
Objective
We performed a randomized, double-blind, double-dummy, crossover study to compare acute non-motor changes between intermittent oral immediate-release carbidopa/levodopa (LC-IR) and LCIG.
Methods
After > 12-h OFF, thirteen PD patients chronically treated with LCIG and without history of non-motor swings, were allocated to receive first, LCIG infusion plus three oral doses of placebo, or placebo infusion plus three oral doses of LC-IR. Over-encapsulated oral medication (LC-IR or placebo) was administered every 2 h. We monitored plasmatic levels of levodopa, motor status (UPDRS-III), mood, anxiety, and frontal functions at baseline (0-h) and hourly after each oral challenge.
Results
Repeated-measures ANOVAs showed significant group by treatment interaction indicating more fluctuations of levodopa plasma levels with LC-IR. No significant interactions were seen in the temporal profile of motor status, anxiety, mood and cognition. However, point-to-point parametric and nonparametric tests showed a significant more marked and more sustained improvement in anxiety scores under LCIG. A significant improvement of mood and verbal fluency was seen a + 3-h only under LCIG.
Discussion
Our sample of advanced PD patients exhibited moderate but significant non-motor fluctuations. LCIG was associated with a more favorable profile of acute affective and cognitive fluctuations that was particularly expressed at the first part of the infusion curve.




Similar content being viewed by others
References
Olanow CW (2019) Levodopa is the best symptomatic therapy for PD: nothing more, nothing less. Mov Disord 34(6):812–815. https://doi.org/10.1002/mds.27690
Verschuur CVM, Suwijn SR, Boel JA, Post B, Bloem BR, van Hilten JJ, van Laar T, Tissingh G, Munts AG, Deuschl G, Lang AE, Dijkgraaf MGW, de Haan RJ, de Bie RMA (2019) Randomized delayed-start trial of levodopa in Parkinson's disease. N Eng J Medi 380(4):315–324. https://doi.org/10.1056/NEJMoa1809983
Espay AJ, Morgante F, Merola A, Fasano A, Marsili L, Fox SH, Bezard E, Picconi B, Calabresi P, Lang AE (2018) Levodopa-induced dyskinesia in Parkinson disease: current and evolving concepts. Ann Neurol 84(6):797–811. https://doi.org/10.1002/ana.25364
Schrag A, Quinn N (2000) Dyskinesias and motor fluctuations in Parkinson's disease. A community-based study. Brain 123(Pt 11):2297–2305. https://doi.org/10.1093/brain/123.11.2297
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16(3):448–458. https://doi.org/10.1002/mds.1090
Nutt JG, Chung KA, Holford NH (2010) Dyskinesia and the antiparkinsonian response always temporally coincide: a retrospective study. Neurology 74(15):1191–1197. https://doi.org/10.1212/WNL.0b013e3181d90050
Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR (2011) The impact of non-motor symptoms on health-related quality of life of patients with Parkinson's disease. Mov Disord 26(3):399–406. https://doi.org/10.1002/mds.23462
Hillen ME, Sage JI (1996) Nonmotor fluctuations in patients with Parkinson's disease. Neurology 47(5):1180–1183. https://doi.org/10.1212/wnl.47.5.1180
Nissenbaum H, Quinn NP, Brown RG, Toone B, Gotham AM, Marsden CD (1987) Mood swings associated with the 'on-off' phenomenon in Parkinson's disease. Psychol Med 17(4):899–904. https://doi.org/10.1017/s0033291700000702
Racette BA, Hartlein JM, Hershey T, Mink JW, Perlmutter JS, Black KJ (2002) Clinical features and comorbidity of mood fluctuations in Parkinson's disease. J Neuropsychiatry Clin Neurosci 14(4):438–442. https://doi.org/10.1176/jnp.14.4.438
Riley DE, Lang AE (1993) The spectrum of levodopa-related fluctuations in Parkinson's disease. Neurology 43(8):1459–1464. https://doi.org/10.1212/wnl.43.8.1459
Storch A, Schneider CB, Wolz M, Sturwald Y, Nebe A, Odin P, Mahler A, Fuchs G, Jost WH, Chaudhuri KR, Koch R, Reichmann H, Ebersbach G (2013) Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology 80(9):800–809. https://doi.org/10.1212/WNL.0b013e318285c0ed
van der Velden RMJ, Broen MPG, Kuijf ML, Leentjens AFG (2018) Frequency of mood and anxiety fluctuations in Parkinson's disease patients with motor fluctuations: a systematic review. Mov Disord 33(10):1521–1527. https://doi.org/10.1002/mds.27465
Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, Poncet M, Cherif AA (2002) Nonmotor fluctuations in Parkinson's disease: frequent and disabling. Neurology 59(3):408–413. https://doi.org/10.1212/wnl.59.3.408
Gallagher DA, Lees AJ, Schrag A (2010) What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov Disord 25(15):2493–2500. https://doi.org/10.1002/mds.23394
Kim A, Kim HJ, Shin CW, Kim Y, Jang M, Jung YJ, Lee WW, Park H, Jeon B (2018) Emergence of non-motor fluctuations with reference to motor fluctuations in Parkinson's disease. Parkinsonism Relat Disord 54:79–83. https://doi.org/10.1016/j.parkreldis.2018.04.020
Delpont B, Lhommee E, Klinger H, Schmitt E, Bichon A, Fraix V, Castrioto A, Quesada JL, Pelissier P, Kistner A, Carnicella S, Luscher C, Broussolle E, Pollak P, Thobois S, Krack P (2017) Psychostimulant effect of dopaminergic treatment and addictions in Parkinson's disease. Mov Disord 32(11):1566–1573. https://doi.org/10.1002/mds.27101
Vazquez A, Jimenez-Jimenez FJ, Garcia-Ruiz P, Garcia-Urra D (1993) “Panic attacks” in Parkinson’s disease. A long-term complication of levodopa therapy. Acta Neurol Scand 87(1):14–18
Kulisevsky J, Garcia-Sanchez C, Berthier ML, Barbanoj M, Pascual-Sedano B, Gironell A, Estevez-Gonzalez A (2000) Chronic effects of dopaminergic replacement on cognitive function in Parkinson's disease: a two-year follow-up study of previously untreated patients. Mov Disord 15(4):613–626. https://doi.org/10.1002/1531-8257(200007)15:4<613:aid-mds1005>3.0.co;2-f
Martinez-Fernandez R, Schmitt E, Martinez-Martin P, Krack P (2016) The hidden sister of motor fluctuations in Parkinson's disease: a review on nonmotor fluctuations. Mov Disord 31(8):1080–1094. https://doi.org/10.1002/mds.26731
Schmitt E, Krack P, Castrioto A, Klinger H, Bichon A, Lhommee E, Pelissier P, Fraix V, Thobois S, Moro E, Martinez-Martin P (2018) The neuropsychiatric fluctuations scale for Parkinson’s disease: a pilot study. Mov Disord Clin Practice 5(3):265–272. https://doi.org/10.1002/mdc3.12607
Stacy M, Bowron A, Guttman M, Hauser R, Hughes K, Larsen JP, LeWitt P, Oertel W, Quinn N, Sethi K, Stocchi F (2005) Identification of motor and nonmotor wearing-off in Parkinson’s disease: comparison of a patient questionnaire versus a clinician assessment. Mov Disord 20(6):726–733. https://doi.org/10.1002/mds.20383
Kulisevsky J, Pascual-Sedano B, Barbanoj M, Gironell A, Pagonabarraga J, Garcia-Sanchez C (2007) Acute effects of immediate and controlled-release levodopa on mood in Parkinson's disease: a double-blind study. Mov Disord 22(1):62–67. https://doi.org/10.1002/mds.21205
Maricle RA, Nutt JG, Valentine RJ, Carter JH (1995) Dose-response relationship of levodopa with mood and anxiety in fluctuating Parkinson's disease: a double-blind, placebo-controlled study. Neurology 45(9):1757–1760. https://doi.org/10.1212/wnl.45.9.1757
Richard IH, Frank S, LaDonna KA, Wang H, McDermott MP, Kurlan R (2005) Mood fluctuations in Parkinson's disease: a pilot study comparing the effects of intravenous and oral levodopa administration. Neuropsychiatric Dis Treat 1(3):261–268
Cools R (2006) Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev 30(1):1–23. https://doi.org/10.1016/j.neubiorev.2005.03.024
Kulisevsky J (2000) Role of dopamine in learning and memory: implications for the treatment of cognitive dysfunction in patients with Parkinson's disease. Drugs Aging 16(5):365–379. https://doi.org/10.2165/00002512-200016050-00006
Gotham AM, Brown RG, Marsden CD (1988) 'Frontal' cognitive function in patients with Parkinson's disease 'on' and 'off' levodopa. Brain 111(Pt 2):299–321. https://doi.org/10.1093/brain/111.2.299
Poewe W, Berger W, Benke T, Schelosky L (1991) High-speed memory scanning in Parkinson's disease: adverse effects of levodopa. Ann Neurol 29(6):670–673. https://doi.org/10.1002/ana.410290616
Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW (2000) Probabilistic learning and reversal deficits in patients with Parkinson's disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia 38(5):596–612. https://doi.org/10.1016/s0028-3932(99)00103-7
Kulisevsky J, Avila A, Barbanoj M, Antonijoan R, Berthier ML, Gironell A (1996) Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson's disease patients at different levodopa plasma levels. Brain 119(Pt 6):2121–2132. https://doi.org/10.1093/brain/119.6.2121
Cools R, Barker RA, Sahakian BJ, Robbins TW (2001) Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex 11(12):1136–1143. https://doi.org/10.1093/cercor/11.12.1136
MacDonald PA, MacDonald AA, Seergobin KN, Tamjeedi R, Ganjavi H, Provost JS, Monchi O (2011) The effect of dopamine therapy on ventral and dorsal striatum-mediated cognition in Parkinson's disease: support from functional MRI. Brain 134(Pt 5):1447–1463. https://doi.org/10.1093/brain/awr075
Pascual-Sedano B, Kulisevsky J, Barbanoj M, Garcia-Sanchez C, Campolongo A, Gironell A, Pagonabarraga J, Gich I (2008) Levodopa and executive performance in Parkinson's disease: a randomized study. J Int Neuropsychol Soc 14(5):832–841. https://doi.org/10.1017/S1355617708081010
Antonini A, Poewe W, Chaudhuri KR, Jech R, Pickut B, Pirtosek Z, Szasz J, Valldeoriola F, Winkler C, Bergmann L, Yegin A, Onuk K, Barch D, Odin P (2017) Levodopa-carbidopa intestinal gel in advanced Parkinson's: final results of the GLORIA registry. Parkinsonism Relat Disord 45:13–20. https://doi.org/10.1016/j.parkreldis.2017.09.018
Buongiorno M, Antonelli F, Camara A, Puente V, de Fabregues-Nebot O, Hernandez-Vara J, Calopa M, Pascual-Sedano B, Campolongo A, Valldeoriola F, Tolosa E, Kulisevsky J, Marti MJ (2015) Long-term response to continuous duodenal infusion of levodopa/carbidopa gel in patients with advanced Parkinson disease: the barcelona registry. Parkinsonism Relat Disord 21(8):871–876. https://doi.org/10.1016/j.parkreldis.2015.05.014
Lang AE, Rodriguez RL, Boyd JT, Chouinard S, Zadikoff C, Espay AJ, Slevin JT, Fernandez HH, Lew MF, Stein DA, Odin P, Fung VS, Klostermann F, Fasano A, Draganov PV, Schmulewitz N, Robieson WZ, Eaton S, Chatamra K, Benesh JA, Dubow J (2016) Integrated safety of levodopa-carbidopa intestinal gel from prospective clinical trials. Mov Disord 31(4):538–546. https://doi.org/10.1002/mds.26485
Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13(2):141–149. https://doi.org/10.1016/S1474-4422(13)70293-X
Lopiano L, Modugno N, Marano P, Sensi M, Meco G, Solla P, Gusmaroli G, Tamma F, Mancini F, Quatrale R, Zangaglia R, Bentivoglio A, Eleopra R, Gualberti G, Melzi G, Antonini A (2019) Motor and non-motor outcomes in patients with advanced Parkinson's disease treated with levodopa/carbidopa intestinal gel: final results of the GREENFIELD observational study. J Neurol 266(9):2164–2176. https://doi.org/10.1007/s00415-019-09337-6
Isacson D, Bingefors K, Kristiansen IS, Nyholm D (2008) Fluctuating functions related to quality of life in advanced Parkinson disease: effects of duodenal levodopa infusion. Acta Neurol Scand 118(6):379–386. https://doi.org/10.1111/j.1600-0404.2008.01049.x
Dafsari HS, Martinez-Martin P, Rizos A, Trost M, Dos Santos Ghilardi MG, Reddy P, Sauerbier A, Petry-Schmelzer JN, Kramberger M, Borgemeester RWK, Barbe MT, Ashkan K, Silverdale M, Evans J, Odin P, Fonoff ET, Fink GR, Henriksen T, Ebersbach G, Pirtosek Z, Visser-Vandewalle V, Antonini A, Timmermann L, Ray Chaudhuri K, Europar, the International P, Movement Disorders Society Non-Motor Parkinson's Disease Study G ( 2019) EuroInf 2: Subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson's disease. Mov Disord 34(3):353–365. https://doi.org/10.1002/mds.27626
Nyholm D, Askmark H, Gomes-Trolin C, Knutson T, Lennernas H, Nystrom C, Aquilonius SM (2003) Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained-release tablets. Clin Neuropharmacol 26(3):156–163. https://doi.org/10.1097/00002826-200305000-00010
Othman AA, Rosebraugh M, Chatamra K, Locke C, Dutta S (2017) Levodopa-carbidopa intestinal gel pharmacokinetics: lower variability than oral levodopa-carbidopa. J Parkinson's Dis 7(2):275–278. https://doi.org/10.3233/JPD-161042
Catalan MJ, Antonini A, Calopa M, Bajenaru O, de Fabregues O, Minguez-Castellanos A, Odin P, Garcia-Moreno JM, Pedersen SW, Pirtosek Z, Kulisevsky J (2017) Can suitable candidates for levodopa/carbidopa intestinal gel therapy be identified using current evidence? eNeurologicalSci 8:44–53. https://doi.org/10.1016/j.ensci.2017.06.004
Volkmann J, Albanese A, Antonini A, Chaudhuri KR, Clarke CE, de Bie RM, Deuschl G, Eggert K, Houeto JL, Kulisevsky J, Nyholm D, Odin P, Ostergaard K, Poewe W, Pollak P, Rabey JM, Rascol O, Ruzicka E, Samuel M, Speelman H, Sydow O, Valldeoriola F, van der Linden C, Oertel W (2013) Selecting deep brain stimulation or infusion therapies in advanced Parkinson's disease: an evidence-based review. J Neurol 260(11):2701–2714. https://doi.org/10.1007/s00415-012-6798-6
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 25(15):2649–2653. https://doi.org/10.1002/mds.23429
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170. https://doi.org/10.1002/mds.22340
Pagonabarraga J, Kulisevsky J, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A (2008) Parkinson's disease-cognitive rating scale: a new cognitive scale specific for Parkinson's disease. Mov Disord 23(7):998–1005. https://doi.org/10.1002/mds.22007
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
Starkstein SE, Merello M, Jorge R, Brockman S, Bruce D, Power B (2009) The syndromal validity and nosological position of apathy in Parkinson's disease. Mov Disord 24(8):1211–1216. https://doi.org/10.1002/mds.22577
Marras C, Troster AI, Kulisevsky J, Stebbins GT (2014) The tools of the trade: a state of the art "How to Assess Cognition" in the patient with Parkinson's disease. Mov Disord 29(5):584–596. https://doi.org/10.1002/mds.25874
Lezak MHD, Bigler E, Tranel D (2012) Neuropsychological assessment. Oxford University Press, Oxford
Sternberg S (1966) High-speed scanning in human memory. Science 153(3736):652–654. https://doi.org/10.1126/science.153.3736.652
Fernandez HH, Standaert DG, Hauser RA, Lang AE, Fung VS, Klostermann F, Lew MF, Odin P, Steiger M, Yakupov EZ, Chouinard S, Suchowersky O, Dubow J, Hall CM, Chatamra K, Robieson WZ, Benesh JA, Espay AJ (2015) Levodopa-carbidopa intestinal gel in advanced Parkinson's disease: final 12-month, open-label results. Mov Disord 30(4):500–509. https://doi.org/10.1002/mds.26123
Black KJ, Hershey T, Hartlein JM, Carl JL, Perlmutter JS (2005) Levodopa challenge neuroimaging of levodopa-related mood fluctuations in Parkinson's disease. Neuropsychopharmacology 30(3):590–601. https://doi.org/10.1038/sj.npp.1300632
Christopher L, Marras C, Duff-Canning S, Koshimori Y, Chen R, Boileau I, Segura B, Monchi O, Lang AE, Rusjan P, Houle S, Strafella AP (2014) Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson's disease with mild cognitive impairment. Brain 137(Pt 2):565–575. https://doi.org/10.1093/brain/awt337
Acknowledgements
We are grateful to all patients and caregivers for their generous participation in the study. This study was partially supported by FIS Grant PI15/00962; Rio Hortega CM17/00209 and CIBERNED (Instituto de Salud Carlos III, Spain); La Marató de TV3, Expedient 20142910, 2014/U/477, and an unrestrictive research grant from Abbvie.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
J Kulisevsky received compensation for consultancy and speaker activities from UCB, AbbVie, Neuroderm, Teva, Roche and Zambon. He received research support from Teva, Zambon, Abbvie, Ciberned and Carlos III Institute Research Grant FIS PI18/0717; S Martinez-Horta received compensation for speaker activities from UCB, AbbVie and Roche. He received research support from the Huntington's Disease Society of America; H Bejr-kasem received compensation for speaker activities in scientific meetings supported by Zambon, and non-financial support for congress attendance from Abbvie, Zambon and Allergan.I Aracil-Bolaños has received research support from Carlos III Institute Research Grant CM19/00156 G; B Pascual-Sedano received compensation for consultancy from Ferrer and speaker activities from AbbVie, UCB and Teva; C Izquierdo received compensation for speaker activities from UCB and Abbvie; A Campolongo received compensation for consultancy and speaker activities from UCB, AbbVie and Teva; J Pagonabarraga has received honoraria as Speaker or as member of Advisory Board from Zambon, UCB, Abbvie, Allergan and Ipsen; J Marín received compensation for consultancy and speaker activities from UCB; J Perez-Perez, O de Fàbregues, V Puente, A Crespo-Cuevas and M Calopa have no conflict of interest to declare.
Ethical standard
The study has been approved by the ethical committee of sant Pau Hospital (Barcelona, Spain). Written informed consent was obtained from all participants and the study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Rights and permissions
About this article
Cite this article
Kulisevsky, J., Bejr-Kasem, H., Martinez-Horta, S. et al. Subclinical affective and cognitive fluctuations in Parkinson's disease: a randomized double-blind double-dummy study of Oral vs. Intrajejunal Levodopa. J Neurol 267, 3400–3410 (2020). https://doi.org/10.1007/s00415-020-10018-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10018-y