Abstract
Objective
Niemann Pick disease type C (NPC) is a rare progressive neurovisceral lysosomal disorder caused by autosomal recessive mutations in the NPC1 or NPC2 genes. 18F-fluorodeoxyglucose (FDG) is a positron-emitting glucose analogue for non-invasive imaging of brain metabolism. FDG PET is commonly used for dementia imaging but its specific application to NPC is rarely described.
Methods
This is a retrospective study of all baseline brain FDG PET performed for NPC patients. Images were assessed using a normal database statistical comparison of metabolic changes expressed in standard deviations and three-dimensional Stereotactic Surface Projection maps. Typical hypometabolic patterns in NPC were identified. We further investigated any correlation between the degree of regional brain hypometabolism and the Iturriaga clinical severity scale.
Results
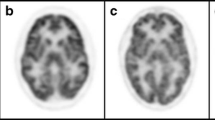
Brain FDG PET images of 14 adolescent–adult NPC patients were analysed, with mean age of 35 years. We found significant frontal lobe hypometabolism in 12 patients (86%), thalamic hypometabolism in eight patients (57%) and variable parietal lobe hypometabolism in 13 patients (93%). Hypometabolic changes were usually bilateral and symmetric. Ten out of 13 ataxic patients showed cerebellar or thalamic hypometabolism (sensitivity 77%, specificity 100%). Linear regression analysis showed frontal lobe hypometabolism to have the best correlation with the Iturriaga clinical scale (R2 = 0.439; p = 0.01).
Conclusions
We found bilateral symmetric hypometabolism of the frontal lobes, thalami and parietal lobes (especially posterior cingulate gyrus) to be typical of adolescent–adult NPC. Ataxia was commonly associated with cerebellar or thalamic hypometabolism. Frontal lobe hypometabolism showed the best inverse correlation with clinical severity.



Similar content being viewed by others
References
Patterson MC, Clayton P, Gissen P et al (2017) Recommendations for the detection and diagnosis of Niemann–Pick disease type C: an update. Neurol Clin Pract 7:499–511
Santos-Lozano A, García DV, Sanchis-Gomar F et al (2015) Niemann–Pick disease treatment: a systematic review of clinical trials. Ann Transl Med 3:1–9
Geberhiwot T, Moro A, Dardis A et al (2018) Consensus clinical management guidelines for Niemann–Pick disease type C. Orphanet J Rare Dis 13:1–19
Iturriaga C, Pineda M, Fernández-Valero EM, Vanier MT, Coll MJ (2006) Niemann–Pick C disease in Spain: clinical spectrum and development of a disability scale. J Neurol Sci 249:1–6
Yanjanin NM, Vélez JI, Gropman A et al (2010) Linear clinical progression, independent of age of onset, in Niemann–Pick disease, type C. Am J Med Genet Part B Neuropsychiatr Genet 153:132–140
Cortina-Borja M, Te Vruchte D, Mengel E et al (2018) Annual severity increment score as a tool for stratifying patients with Niemann–Pick disease type C and for recruitment to clinical trials. Orphanet J Rare Dis 13:1–16
Walterfang M, Patenaude B, Abel LA et al (2013) Subcortical volumetric reductions in adult Niemann–Pick disease type C: a cross-sectional study. Am J Neuroradiol 34:1334–1340
Bowman EA, Walterfang M, Abel L, Desmond P, Fahey M, Velakoulis D (2015) Longitudinal changes in cerebellar and subcortical volumes in adult-onset Niemann–Pick disease type C patients treated with miglustat. J Neurol 262:2106–2114
Abel LA, Bowman EA, Velakoulis D et al (2012) Saccadic eye movement characteristics in adult Niemann–Pick type C disease: relationships with disease severity and brain structural measures. PLoS ONE 7:e50947
Tripathi M, Tripathi M, Damle N et al (2014) Differential diagnosis of neurodegenerative dementias using metabolic phenotypes on F-18 FDG PET/CT. Neuroradiol J 27:13–21
Morbelli S, Garibotto V, Van De Giessen E et al (2015) A cochrane review on brain [18F]FDG PET in dementia: limitations and future perspectives. Eur J Nucl Med Mol Imaging 42:1487–1491
Walterfang M, Fahey M, Desmond P et al (2010) White and gray matter alterations in adults with Niemann–Pick disease type C: a cross-sectional study. Neurology 75:49–56
Villemagne VL, Velakoulis D, Doré V et al (2019) Imaging of tau deposits in adults with Niemann–Pick type C disease: a case-control study. Eur J Nucl Med Mol Imaging 46:1132–1138
Sévin M, Lesca G, Baumann N et al (2007) The adult form of Niemann–Pick disease type C. Brain 130:120–133
Huang JY, Peng SF, Yang CC, Yen KY, Tzen KY, Yen RF (2011) Neuroimaging findings in a brain with Niemann–Pick type C disease. J Formos Med Assoc 110:537–542
Battisti C, Tarugi P, Dotti MT et al (2003) Adult onset Niemann–Pick type C disease: a clinical, neuroimaging and molecular genetic study. Mov Disord 18:1405–1409
Trendelenburg G, Vanier MT, Maza S et al (2006) Niemann–Pick type C disease in a 68-year-old patient. J Neurol Neurosurg Psychiatry 77:997–998
Kumar A, Chugani HT (2011) Niemann–Pick disease type C: unique 2-deoxy-2[18F] fluoro-d-glucose PET abnormality. Pediatr Neurol 44:57–60
Benussi A, Alberici A, Premi E et al (2015) Phenotypic heterogeneity of Niemann–Pick disease type C in monozygotic twins. J Neurol 262:642–647
Rego T, Farrand S, Goh AMY et al (2019) Psychiatric and cognitive symptoms associated with Niemann–Pick type C disease: neurobiology and management. CNS Drugs 33:125–142
Walterfang M, Fietz M, Fahey M et al (2006) The neuropsychiatry of Niemann–Pick type C disease in adulthood. J Neuropsychiatry Clin Neurosci 18:158–170
Walterfang M, Di Biase MA, Cropley VL et al (2020) Imaging of neuroinflammation in adult Niemann–Pick type C disease. Neurology 94:e1716–e1725
Diehl-Schmid J, Licata A, Goldhardt O et al (2019) FDG-PET underscores the key role of the thalamus in frontotemporal lobar degeneration caused by C9ORF72 mutations. Transl Psychiatry 9:54
Jeong Y, Cho SS, Park JM et al (2005) 18F-FDG PET findings in frontotemporal dementia: an SPM analysis of 29 patients. J Nucl Med 46:233–239
Shimogori K, Doden T, Oguchi K, Hashimoto T (2017) Thalamic and cerebellar hypermetabolism and cortical hypometabolism during absence status epilepticus. BMJ Case Rep 2017:bcr2017220139
Prieto E, Domínguez-Prado I, Riverol M et al (2015) Metabolic patterns in prion diseases: an FDG PET voxel-based analysis. Eur J Nucl Med Mol Imaging 42:1522–1529
Choo IH, Lee DY, Youn JC et al (2007) Topographic patterns of brain functional impairment progression according to clinical severity staging in 116 Alzheimer disease patients: FDG-PET study. Alzheimer Dis Assoc Disord 21:77–84
Kato T, Inui Y, Nakamura A, Ito K (2016) Brain fluorodeoxyglucose (FDG) PET in dementia. Ageing Res Rev 30:73–84
Whitwell JL, Graff-Radford J, Singh TD et al (2017) 18F-FDG PET in posterior cortical atrophy and dementia with Lewy bodies. J Nucl Med 58:632–638
Masingue M, Adanyeguh I, Nadjar Y, Sedel F, Galanaud D, Mochel F (2017) Evolution of structural neuroimaging biomarkers in a series of adult patients with Niemann–Pick type C under treatment. Orphanet J Rare Dis 12:22
Bremova-Ertl T, Sztatecsny C, Brendel M et al (2020) Clinical, ocular motor, and imaging profile of Niemann–Pick type C heterozygosity. Neurology 94:e1702–e1715
Piroth T, Boelmans K, Amtage F et al (2017) Adult-onset Niemann–Pick disease type C: rapid treatment initiation advised but early diagnosis remains difficult. Front Neurol 8:8–11
Chiba Y, Komori H, Takei S et al (2014) Niemann–Pick disease type C1 predominantly involving the frontotemporal region, with cortical and brainstem Lewy bodies: an autopsy case. Neuropathology 34:49–57
Bostan AC, Strick PL (2010) The cerebellum and basal ganglia are interconnected. Neuropsychol Rev 20:261–270
Solomon DH, Barohn RJ, Bazan C, Grissom J (1994) The thalamic ataxia syndrome. Neurology 44:810–814
Elleder M, Jirásek A (1981) Neuropathology of various types of Niemann–Pick disease. Acta Neuropathol Suppl 7:201–203
Berti V, Mosconi L, Pupi A (2014) Brain: normal variations and benign findings in fluorodeoxyglucose-PET/computed tomography imaging. PET Clin 9:129–140
Jeong YJ, Yoon HJ, Kang DY (2017) Assessment of change in glucose metabolism in white matter of amyloid-positive patients with Alzheimer disease using F-18 FDG PET. Medicine (Baltimore) 96:e9042
Vanier MT (2010) Niemann–Pick disease type C. Orphanet J Rare Dis 5:16
Acknowledgements
We thank Dr Digsu Koye (PhD) from Melbourne EpiCentre for his assistance with statistical analysis.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no competing interests.
Ethical approval
This study was approved by the Melbourne Health Research and Ethics Committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects provided informed consent for the study.
Rights and permissions
About this article
Cite this article
Lau, T.Y., Kao, Y.H., Toh, H.B. et al. Brain hypometabolic changes in 14 adolescent–adult patients with Niemann–Pick disease type C assessed by 18F-fluorodeoxyglucose positron emission tomography. J Neurol 268, 3878–3885 (2021). https://doi.org/10.1007/s00415-021-10535-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10535-4