Abstract
The PERaMpanel pooled analysIs of effecTiveness and tolerability (PERMIT) study was a pooled analysis of data from 44 real-world studies from 17 countries, in which people with epilepsy (PWE; focal and generalized) were treated with perampanel (PER). Retention and effectiveness were assessed after 3, 6, and 12 months, and at the last visit (last observation carried forward). Effectiveness assessments included 50% responder rate (≥ 50% reduction in seizure frequency from baseline) and seizure freedom rate (no seizures since at least the prior visit); in PWE with status epilepticus, response was defined as seizures under control. Safety and tolerability were assessed by evaluating adverse events (AEs) and discontinuation due to AEs. The Full Analysis Set included 5193 PWE. Retention, effectiveness and safety/tolerability were assessed in 4721, 4392 and 4617, respectively. Retention on PER treatment at 3, 6, and 12 months was 90.5%, 79.8%, and 64.2%, respectively. Mean retention time on PER treatment was 10.8 months. The 50% responder rate was 58.3% at 12 months and 50.0% at the last visit, and the corresponding seizure freedom rates were 23.2% and 20.5%, respectively; 52.7% of PWE with status epilepticus responded to PER treatment. Overall, 49.9% of PWE reported AEs and the most frequently reported AEs (≥ 5% of PWE) were dizziness/vertigo (15.2%), somnolence (10.6%), irritability (8.4%), and behavioral disorders (5.4%). At 12 months, 17.6% of PWEs had discontinued due to AEs. PERMIT demonstrated that PER is effective and generally well tolerated when used to treat people with focal and/or generalized epilepsy in everyday clinical practice.
Similar content being viewed by others
Introduction
Perampanel (PER) is the first-in-class, highly selective, non-competitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist that inhibits the postsynaptic binding of glutamate by selectively targeting AMPA receptors, which are mainly located on postsynaptic neurons [1,2,3,4]. PER is widely indicated for both focal-onset seizures and generalized-onset tonic–clonic seizures in patients with idiopathic generalized epilepsy (IGE). Its approved use in different age groups, and as monotherapy or adjunctive therapy, varies between countries and regions [5,6,7].
Approval for the treatment of focal-onset seizures was based primarily on the results of three Phase III randomized, double-blind, placebo-controlled trials [8,9,10] and one pediatric open-label Phase III trial [11]. Approval for the treatment of generalized-onset tonic–clonic seizures in IGE was based on the findings of one Phase III, randomized, double-blind, placebo-controlled trial [12]. Real-world clinical practice data complement evidence from clinical trials by providing information on people with epilepsy (PWE) who are more diverse in terms of clinical characteristics than those recruited for clinical trials [13,14,15]. In addition, they provide pragmatic information on the dosing and titration schedules employed in clinical practice, which are individualized and applied on a patient-by-patient basis, rather than according to a pre-defined clinical trial protocol [14]. The objective of this study was to investigate the effectiveness, safety and tolerability of PER when used in everyday clinical practice.
Methods
Study design
The PERaMpanel pooled analysIs of effecTiveness and tolerability (PERMIT) study was a pooled analysis of individual patient data from real-world prospective, retrospective and cross sectional studies and work groups in which people with focal and generalized epilepsy were treated with PER. The studies were identified by a systematic PubMed literature search, supported by searches of abstracts from key epilepsy congresses from 2012 to December 2019. There were no exclusion criteria for the studies or work groups included in the analysis in terms of the epilepsy type of those studied and/or the number of prior antiseizure medications (ASMs) they had received. The only exclusion criterion for the overall study was that the principal investigator of the respective study did not agree to participate in the pooled analysis. De-identified data from individual PWE were pooled together for baseline number of seizures, type of epilepsy/seizures, prior ASMs, dosage, effectiveness at various time points, and adverse events (AEs). Effectiveness was assessed after 3, 6, and 12 months of PER treatment and at final follow-up (i.e. the last observation of each PWE, independent of the timepoint when it occurred [last observation carried forward]; defined as ‘last visit’). Safety and tolerability were assessed for the duration of PER treatment.
Each study included in PERMIT was approved by its own independent ethics committee, which was subsequently informed, if needed by local legislation, about the PERMIT pooled analysis.
Study population
PERMIT included PWE from studies and work groups conducted in 17 countries in Europe, Asia, North America, the Middle East and Australasia (Table 1). Details of the specific inclusion/exclusion criteria used in the individual studies have been published or presented previously [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. Studies included in the analysis employed broad inclusion/exclusion criteria, to be representative of PWE encountered in clinical practice. All PWE from these studies who initiated PER for the treatment of epilepsy were included in the pooled analysis. PWE were excluded if records contained insufficient data for analysis. PWE from publications by the same authors and the same geographic areas were compared, and PWE with the same baseline characteristics, treatment start/end date, information at follow-up visits, and treatment completion were excluded, in order to ensure that there were no duplicate data.
Study assessments
Retention was assessed after 3, 6 and 12 months of PER treatment. Long-term retention (defined as > 12 months) was also assessed for those studies that reported it. Effectiveness was assessed after 3, 6 and 12 months and at the last visit. In individuals with focal or generalized seizures, effectiveness assessments included change from baseline in seizure frequency (by seizure type), 50% responder rate (response defined as ≥ 50% reduction in seizure frequency from baseline), seizure freedom rate (seizure freedom defined as no seizures since at least the prior visit), and the proportion of PWE with worsened seizure frequency. At baseline (i.e., prior to PER initiation), monthly seizure frequency was defined based on the criteria used for each individual study. At other timepoints, monthly seizure frequency was based on the number of seizures experienced since the previous visit. For the final assessment, monthly seizure frequency was based on the last visit, which could have been at 3, 6, or 12 months; therefore, seizure frequency at the last visit was based on the number of seizures experienced during at least the previous 3 months. In individuals with status epilepticus, effectiveness was assessed as responder rate, where response was defined as seizures under control; this meant that the patient responded to PER treatment and their seizures remained under control with its use (although the duration of control was not reported). Safety and tolerability were assessed by evaluating the number and type of AEs, AEs leading to discontinuation, psychiatric AEs, and psychiatric AEs leading to discontinuation. Additional assessments included evaluation of information relating to PER dosing and titration, and changes in the number of concomitant ASMs between baseline and the last visit.
Statistical analyses
Analysis populations
The Full Analysis Set (FAS) comprised all PWE who were treated with PER. The Retention Population included PWE from the FAS population whose PER status was known at some point during the first 12 months after starting treatment (including those with ongoing PER treatment at 12 months, those who stopped PER prior to 12 months, and those lost to follow-up or end of study follow-up prior to 12 months). The Effectiveness Population included PWE from the FAS population who had at least one effectiveness measurement available. The Tolerability Population included PWE from the FAS population for whom data on AEs were available.
Statistical considerations and methods
There was great heterogeneity in the particular objectives of each study included in the pooled analysis and therefore in the information that each study reported. The current study attempted to combine the reported information in the most complete and harmonized way possible. If patients had effectiveness assessed, their measurements were collected until they discontinued or were lost to follow-up. Missing data were not imputed, except in cross-sectional studies, in which the last visit datum was captured to include it in the established cut-off points (3, 6 or 12 months). When the observation timepoint of the study did not match the established cut-off points, the following allocations were made: observations performed between 1.5–4.5 months were allocated to the 3-month visit; those performed between 4.5–9 months were allocated to the 6-month visit; and those performed between 9–15 months were allocated to the 12-month visit. A ‘final’ variable was created in which the last observation of each PWE was included (last observation carried forward), independently of the moment when it occurred (defined as ‘last visit’). Since the studies included in PERMIT did not have a common objective and varied in terms of patient selection criteria, no hypothesis was defined, meta-analysis of individualized PWE data was not conducted, and the individual studies were not treated as clusters.
A descriptive analysis of recorded quantitative and qualitative variables was performed. Quantitative variables were described as mean, standard deviation (SD), median, minimum and maximum values, together with the number of valid cases and confidence intervals (CIs) or interquartile range (25th percentile to 75th percentile). Qualitative variables were described as absolute frequencies and percentages. Data were not available for all PWE at every time point; therefore, for each variable, the total number of PWE for whom the data in question were available was stated and this value was used as the denominator for frequency analyses. Retention (on PER treatment) was studied within the first 12 months of follow-up using Kaplan–Meier methodology. Variation in the number of seizures per month between baseline and the last visit was assessed using the Wilcoxon signed-rank test and variation in the type of seizures was assessed using McNemar’s test. The signification level was established at 5%. Statistical package SPSS 25.0 was used for all analyses.
Bivariate and multivariate analyses
Binary logistic regression analyses were performed to study possible factors associated with retention, effectiveness (seizure freedom, response) and tolerability. Potential relationships between baseline characteristics (subject- and therapy-related factors) and the dependent variables (retention, seizure freedom, response, tolerability) were determined by bivariable methods, using the Chi-squared test, Student’s t-test or Mann–Whitney U test, as appropriate, to determine statistical significance. Baseline characteristics included sex, age, age at epilepsy onset, duration of epilepsy, etiology, presence of learning disability, presence of psychiatric comorbidity, seizure type, use of PER as early or late add-on therapy, use of PER monotherapy, rate of PER titration (fast [2 mg/week]/slow [less than 2 mg/week]), PER dosage (≤ 4 mg/day, ≤ 6 mg/day), number of previous ASMs, and number and types of concomitant ASMs. Baseline characteristics with a p-value of < 0.10 were pre-selected to construct exploratory multivariable binary logistic regression models for retention, effectiveness and tolerability.
Results
Information was gathered from 5200 PWE who had initiated treatment with PER. Two PWE were excluded because they never started treatment and five PWE because they were included in two studies. The final FAS therefore included 5193 individual PWE from a total of 44 real-world studies/work groups from a wide range of countries, details of which are presented in Table 1 [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56].
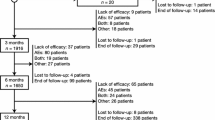
Disposition was assessed for the Retention Population, which included 4721 PWE (Fig. 1). Overall, 2698 PWE (57.1%) completed at least 12 months of PER treatment. The Effectiveness Population included 4392 PWE and the Tolerability Population included 4617 PWE.
Study population
Demographic and baseline characteristics are presented in Table 2. In the FAS, 50.5% of PWE were female, the mean (SD) age was 39.7 (16.2) years and the majority of PWE (85.6%) were aged ≥ 18 to < 65 years. Mean (SD) duration of epilepsy was 23.5 (16.0) years and 52.7% of PWE had a structural etiology (International League Against Epilepsy 2017 classification [57]). The median number of previous ASMs PWE had received was 4.0 (range 0‒19; mean 4.9; SD, 3.9) and the median number of concomitant ASMs at baseline was 2.0 (range 0‒7; mean 2.2; SD, 1.2). Demographic and baseline characteristics of PWE in the retention, effectiveness and tolerability populations were generally similar to those of the FAS population (Table 2).
PER treatment and concomitant ASMs (FAS)
The mean (SD) baseline PER dose was 2.4 (1.1) mg/day in the overall population (median 2.0; range, 1–20; n = 2190). At the end of the observation period (last visit), the mean (SD) PER dose was 6.3 (2.6) mg/day (median 6.0; range 1–24; n = 3411). In individuals without status epilepticus, the mean (SD) PER dose was 2.3 (1.1) mg/day (median 2.0; range 1–20; n = 2168) at baseline, and 6.3 (2.6) mg/day (median 6.0; range 1–18; n = 3339) at the last visit. In those with status epilepticus, the mean (SD) PER dose was 6.6 (3.8) mg/day (median 6.0; range 2–24; n = 72). The rate of PER titration was known for 1880 PWE. A fast titration (2 mg/week) was used in 65.5% of PWE (1231/1880) and a slow titration (less than 2 mg/week) was used in 34.5% of PWE (649/1880). The mean (SD) number of concomitant ASMs was 2.2 (1.2) mg/day (median 2.0; range 0–7; n = 4916) at baseline and 2.0 (1.0) mg/day (median 2.0; range 0–6; n = 1832) at the last visit. The proportion of PWE treated with PER monotherapy was 5.6% (269/4816) at baseline and 4.1% (76/1832) at the last visit.
Retention (Retention Population)
Retention on PER treatment at 3, 6, and 12 months was 90.5% (4273/4721), 79.8% (3603/4516), and 64.2% (2698/4201), respectively. The mean retention time on PER treatment was 10.8 months (95% CI 10.6–10.9; Kaplan–Meier analysis; Fig. 2). It was not possible to estimate the median retention time, due to the high number of censored events. Reasons for discontinuation over 12 months were AE(s) (14.3%; 600/4201), lack of efficacy (8.8%; 368/4201), both AE(s) and lack of efficacy (3.3%; 139/4201), seizure worsening (1.2%; 49/4201), other reasons (0.8%; 34/4201; death, n = 9; financial problems, n = 7; pregnancy, n = 4; PWE decision—not otherwise specified, n = 4; disease progression [tumor], n = 4; transferred to another hospital, n = 2; surgery, n = 1; unable to take medicine [pneumonia], n = 1; poor compliance, n = 1; interaction with tuberculosis medication, n = 1) and unknown (7.5%; 313/4201). Over the longer term (> 12 months), retention was 29.5% (1229/4164) and the mean retention time on PER treatment was 18.7 months (95% CI, 17.8–19.5). Reasons for discontinuation over the longer term were lack of efficacy (23.8%; 990/4164), AE(s) (16.4%; 683/4164), both AE(s) and lack of efficacy (4.2%; 173/4164), seizure worsening (1.4%; 60/4164), other reasons (1.1%; 46/4164; death, n = 10; financial problems, n = 8; pregnancy, n = 6; improvement, n = 5; PWE decision—not otherwise specified, n = 4; disease progression [tumor], n = 4; surgery, n = 2; transferred to another hospital, n = 2; accident, n = 1; prior myocardial infarction, n = 1; unable to take medicine [pneumonia], n = 1; poor compliance, n = 1; interaction with tuberculosis medication, n = 1) and unknown (23.6%; 983/4164).
Results of bivariable analysis of the associations of baseline characteristics with retention at 12 months are presented in Supplementary Table 1 (baseline characteristics selected for inclusion in the model to predict retention [i.e. those with p < 0.10] are indicated as shaded cells). Multivariate binary regression analysis revealed that the baseline characteristics that were most associated with likelihood of retention were lower number of previous ASMs (odds ratio [OR] 1.07 [95% CI 1.04–1.10]; p < 0.001) and use of a slow PER titration schedule (OR 2.13 [95% CI 1.68–2.71]) (Fig. 3A). The resulting model had high sensitivity (94.6%) but very low specificity (10.7%).
Multivariate analyses of relationships between baseline characteristics and a retention (Retention Population), b response to PER treatment (Effectiveness Population) and c seizure freedom (Effectiveness Population). Seizure freedom was defined as no seizures since at least the prior visit. Response was defined as ≥ 50% seizure frequency reduction from baseline. ASM antiseizure medication, AUC area under the curve, CI confidence interval, OR odds ratio
Effectiveness (all PWE except those with status epilepticus; Effectiveness Population)
Change in seizure frequency by seizure type
At the time of PER initiation, 96.3% (1825/1896) of PWE presented having had at least one seizure in the past 3 months. The monthly total seizure frequency decreased significantly from a median of 3.0 (mean [SD], 17.2 [60.6]: range 0.1–1120.0) at baseline to 0.7 (mean [SD], 7.3 [22.2]: range 0.0–300.0) at the last visit (|Z|= 21.51; p < 0.001; Wilcoxon signed-rank test) (Fig. 4A). The mean reduction from baseline to the last visit was 57.3% (median, 75.0%). The percentage of individuals with focal seizures (i.e., the percentage of PWE who experienced at least one focal seizure during an assessment period of ≥ 3 months) decreased significantly from 71.8% (1167/1626) at baseline to 51.9% (682/1314) at the last visit (p < 0.001; McNemar’s test). Similarly, the percentage of PWE with focal aware seizures decreased from 30.2% (313/1037) at baseline to 20.1% (173/861) at the last visit (p < 0.001), the percentage of PWE with focal impaired awareness seizures decreased from 55.4% (574/1037) at baseline to 40.3% (347/861) at the last visit (p < 0.001), and the percentage of PWE with focal to bilateral tonic–clonic seizures decreased from 41.6% (431/1037) at baseline to 20.8% (179/861) at the last visit (p < 0.001). There were significant reductions from baseline to the last visit in the monthly frequencies of focal, focal aware, focal impaired awareness, and focal to bilateral tonic–clonic seizures (mean [median] reductions, 46.8% [70.0%], 47.1% [100%], 44.0% [100%], and 65.4% [100%], respectively; p < 0.001 for all [baseline versus last visit]) (Fig. 4B–E).
Median monthly frequencies (with P25 and P75 IQR) at baseline, Month 3, Month 6, Month 12 and the last visit for a total seizures, b focal seizures, c focal aware seizures, d focal impaired awareness seizures, e focal to bilateral tonic–clonic seizures, f primary generalized seizures, g primary generalized tonic–clonic seizures, h generalized tonic seizures, i absence seizures, j myoclonic seizures, and k days with myoclonic seizures. IQR interquartile range, P percentile
The percentage of PWE with primary generalized seizures decreased significantly from 24.5% (399/1626) at baseline to 10.8% (142/1314) at the last visit (p < 0.001; McNemar’s test). The percentage of PWE with primary generalized tonic–clonic seizures decreased from 76.9% (300/390) at baseline to 25.8% (78/302) at the last visit (p < 0.001), the percentage of PWE with generalized tonic seizures decreased from 9.2% (36/390) at baseline to 1.3% (4/302) at the last visit (p = not significant), the percentage of PWE with absence seizures decreased from 20.0% (78/390) at baseline to 11.9% (36/302) at the last visit (p < 0.001), and the percentage of PWE with myoclonic seizures decreased from 30.3% (118/390) at baseline to 11.9% (36/302) at the last visit (p < 0.001). There were significant reductions from baseline to the last visit in the monthly frequencies of generalized, generalized tonic–clonic, generalized tonic, absence and myoclonic seizures, and in the frequency of days with myoclonic seizures (mean [median] reductions, 78.1% [100%], 68.4% [100%], 58.1% [100%], 85.0% [100%], 64.9% [100%], and 64.8% [100%], respectively; p < 0.001 for all except generalized tonic seizures [p = 0.028] [baseline vs. last visit]) (Fig. 4F–K).
Responder rate
The responder rate was 58.3% at 12 months and 50.0% at the last visit (Fig. 5).
Responder rate, seizure freedom rate, and percentage of PWE with worsened seizure frequency (relative to baseline) at Month 3, Month 6, Month 12 and the last visit. Response was defined as ≥ 50% reduction in seizure frequency from baseline. Seizure freedom was defined as no seizures since at least the prior visit; therefore, seizure freedom rates at Month 3, Month 6 and the last visit represent the percentages of PWE who had no seizures for ≥ 3 months, and the seizure freedom rate at Month 12 represents the percentage of PWE who had no seizures for ≥ 6 months. PWE people with epilepsy
Results of bivariable analysis of the associations of baseline characteristics with response during the first 12 months are presented in Supplementary Table 2A (baseline characteristics selected for inclusion in the model to predict response [i.e. those with p < 0.10] are indicated as shaded cells). Multivariate binary regression analysis revealed that the baseline characteristics that were most associated with likelihood of response were higher age of onset of epilepsy (OR 1.01 [95% CI 1.01–1.02]; p < 0.001), presence of a genetic etiology (OR 4.33 [95% CI 3.07–6.11]; p < 0.001), absence of psychiatric comorbidity (OR 1.40 [95% CI 1.06–1.86]; p = 0.020), lower number of previous ASMs (OR 1.14 [95% CI 1.09–1.18]; p < 0.001), and lower number of concomitant ASMs at baseline (OR 1.40 [95% CI 1.24–1.58]; p < 0.001) (Fig. 3B). However, the resulting model had low sensitivity (67.4%) and specificity (73.8%).
Seizure freedom rate
The seizure freedom rate was 23.2% at 12 months and 20.5% at the last visit (Fig. 5). A total of 195 PWE presented with no seizures at every recorded timepoint during follow-up (although in some cases only the last visit was recorded). The duration of observation for these PWE ranged from 2.9 to 14 months. Of these 195 PWE, 157 (80.5%) presented with seizures at baseline and 38 (19.5%) did not present with seizures at baseline.
Results of bivariable analysis of the associations of baseline characteristics with seizure freedom during the first 12 months are presented in Supplementary Table 2B (baseline characteristics selected for inclusion in the model to predict seizure freedom [i.e. those with p < 0.10] are indicated as shaded cells). Multivariate binary regression analysis revealed that the baseline characteristics that were most associated with likelihood of seizure freedom were higher age at onset of epilepsy (OR 1.02 [95% CI 1.01–1.03]; p < 0.001), presence of a genetic etiology (OR 5.87 [95% CI 4.25–8.09]; p < 0.001), absence of psychiatric comorbidity (OR 1.52 [95% CI 1.09–2.11]; p = 0.014), lower number of previous ASMs (OR 1.12 [95% CI 1.06–1.18]; p < 0.001), and lower number of concomitant ASMs at baseline (OR 1.73 [95% CI 1.49–2.00]; p < 0.001) (Fig. 3C). The resulting model had high sensitivity (90.5%) but low specificity (48.1%).
Percentage of PWE with worsened seizure frequency
The percentages of PWE with worsened seizure frequency, relative to baseline, remained generally stable at all timepoints (Fig. 5). The percentage of PWE with worsened seizure frequency was 6.6% at 12 months and 10.1% at the last visit. The percentage of PWE with focal seizures who had worsening seizure frequency was 7.6% at 12 months and 11.2% at the last visit; in those with generalized seizures, the corresponding values were 2.7% and 5.7%, respectively.
Effectiveness in PWE with status epilepticus (Effectiveness Population)
A total of 74 PWE in the Effectiveness Population had status epilepticus. Of these PWE, 39 (52.7%) responded to PER treatment (i.e., PER treatment brought status epilepticus under control).
Safety and tolerability (Tolerability Population)
Overall, 49.9% (2303/4617) of PWE reported AEs at some point during follow-up (Tolerability Population; Table 3). The most frequently reported AEs (≥ 3% of PWE) were dizziness/vertigo (15.2%), somnolence (10.6%), irritability (8.4%), behavioral disorders (5.4%), instability/ataxia (4.1%), and fatigue (3.7%). The incidence of AEs was significantly higher in PWE for whom a fast PER titration was used, compared with those for whom a slow titration was used (61.9% [646/1043] vs. 46.4% [295/636]; χ2 = 9.36; p = 0.002; Chi-square test). Psychiatric AEs were experienced by 21.0% (965/4590) of PWE. There was a significant association between the incidence of psychiatric AEs and the presence of previous psychiatric comorbidity (χ2 = 52.43; p < 0.001; Chi-squared test). Of the 965 PWE with psychiatric AEs, the presence of psychiatric comorbidity was known for 509 PWE, of whom 185 (36.3%) had psychiatric comorbidity and 324 (63.7%) did not. Of the 3625 PWE without psychiatric AEs, the presence of psychiatric comorbidity was known for 1809 PWE, of whom 376 (20.8%) had psychiatric comorbidity and 1433 (79.2%) did not. At 12 months, 17.6% (739/4201) of PWEs had discontinued PER due to AEs. Over the longer term (> 12 months), 20.6% (856/4164) of PWE discontinued PER due to AEs. Overall, 9.6% (414/4294) of PWE discontinued PER due to AEs and had psychiatric AEs (although it was not possible to determine in all PWE whether the withdrawal of PER was exclusively due to the psychiatric AEs). The most frequent psychiatric AEs (≥ 1% of PWE) in those who discontinued PER due to AEs were irritability (3.1%), behavioral disorders (2.8%) and mood disturbance (1.1%). No cases of homicidal ideation were reported.
Results of bivariable analysis of the associations of baseline characteristics with occurrence of AEs are presented in Supplementary Table 3 (baseline characteristics selected for inclusion in the model to predict occurrence of AEs [i.e. those with p < 0.10] are indicated as shaded cells). It was not possible to obtain a multivariate model to predict baseline characteristics associated with the presence/absence of AEs.
Discussion
The results of the PERMIT study demonstrate that PER was effective and generally well tolerated when used to treat focal or generalized epilepsy in everyday clinical practice. After 12 months of PER treatment, 64.2% of PWE were retained on PER, 58.3% had responded to treatment (≥ 50% reduction in seizure frequency from baseline), and 23.2% had been seizure free for at least 6 months. In addition, PER brought seizures under control in 52.7% of PWE with status epilepticus. There were significant reductions from baseline to the last visit in the monthly frequencies of total seizures, all types of focal and generalized seizures, and days with myoclonic seizures. A low proportion of PWE experienced seizure worsening after initiating PER treatment (6.6% at 12 months).
An important finding from the study was that PER was generally well tolerated when used over the long term in clinical practice. Approximately half the population experienced AEs at some point during follow-up, and the most frequently reported AEs (dizziness/vertigo, somnolence, irritability, behavioral disorders, instability/ataxia and fatigue) were consistent with those reported in clinical trials [5, 8,9,10,11,12]. The incidence of AEs was significantly higher in PWE for whom a fast PER titration was used (2 mg/week), compared with those for whom a slow titration was used (less than 2 mg/week), supporting evidence from previous clinical practice studies demonstrating lower incidences of AEs and AEs leading to discontinuation when PER was titrated more slowly than the rigid titration schedules employed in randomized clinical trials [58, 59]. Psychiatric AEs, which were reported as a common side effect in clinical trials (affecting ≥ 1/100 to < 1/10 PWE) [5], were experienced by 21.0% of PWE in PERMIT, and 9.6% of PWE who discontinued PER due to AEs had psychiatric AEs. Since a significant association was found between the incidence of psychiatric AEs and the presence of previous psychiatric comorbidity, the higher incidence of psychiatric AEs observed in PERMIT, in comparison with clinical trials, is likely to reflect the fact that clinical trials typically exclude PWE with psychiatric comorbidities [13, 15], whereas almost a quarter of PWE in PERMIT had psychiatric comorbidities at baseline (23.8%). Indeed, other evidence has indicated that PER-associated psychiatric and behavioral symptoms vary depending on the type of psychiatric and behavioral comorbidities present [60]. Although PER might aggravate some pre-existing psychiatric and behavioral symptoms, other such symptoms may improve with PER therapy [60]. It is noteworthy that there were no cases of homicidal ideation in PERMIT, some cases of which were reported in clinical trials [5, 61], illustrating that an individualized approach to dosage and titration can improve outcomes.
Evidence from clinical trials has shown that PER-associated AEs are more common during initial titration and appear to be dose-related; therefore, individuals should be monitored for these side effects, particularly during titration and at higher doses [62]. The overall incidence of AEs in PERMIT (49.9%) was lower than the rates reported in clinical trials, which ranged from 61.7% to 91.8% [8,9,10,11,12]. This is perhaps surprising, given that PWE treated in clinical practice are more diverse in terms of age and clinical characteristics, have higher levels of comorbidity and associated comedication, and may be more severe and refractory to treatment than those recruited for clinical trials [15]. However, this may in part be explained by the individualized approach to treatment used in clinical practice (in comparison with the defined dosing schedules employed in clinical trials), which is likely to result in improved tolerability. The proportion of PWE who discontinued due to AEs (17.6% after 12 months) was higher than most of the rates reported in clinical trials, which ranged from 2.9% to 19.0%; however, the durations of these trials were only 12–13 weeks [8,9,10,11,12]. PERMIT is the largest pooled analysis of PER clinical practice data conducted to date, with safety/tolerability assessed in over 4600 PWE; a far larger population than the total number of PWE included in PER clinical trials (approximately 1640) [5]. It is therefore reassuring that no new or unexpected safety signals emerged over the long term when PER was used under everyday clinical practice conditions.
Status epilepticus represents one of the most serious neurological emergencies [63] and encompasses a wide variety of subtypes and etiologies [64,65,66,67]. Benzodiazepines are typically used for first-line treatment of early status epilepticus, with intravenous ASMs administered as second-line therapy if seizures progress into established status epilepticus [66, 68,69,70]. ASMs commonly used in this setting include valproate, phenobarbital, phenytoin/fosphenytoin, levetiracetam and lacosamide, although there is no clear evidence for a preferred choice of second-line ASM therapy [66, 68, 69, 71]. Evidence for the use of PER in the treatment of status epilepticus is currently limited. In a systematic review (published in 2018) that included 10 articles and a total of 68 PWE, the rate of seizure control following treatment with PER ranged from 17 to 100% [72]. More recently, a single-center, retrospective, observational study of 75 PWE treated with PER for refractory status epilepticus reported a responder rate of 41.3% (response defined as clear resolution of the ictal pattern and/or seizures within 72 h of PER administration) [73]. In a further cohort study of 81 PWE treated with PER in intensive care for refractory or super-refractory status epilepticus, 33.3% responded to treatment [74]. In PERMIT, which included 74 PWE with status epilepticus from a wide range of clinical practice settings, the responder rate was 52.7%. PERMIT therefore provides valuable additional evidence indicating that PER may be a useful oral ASM therapy for some PWE with status epilepticus.
The large size of the PERMIT cohort allowed exploratory multivariable binary logistic regression analyses to be conducted, in order to try and identify baseline subject- and treatment-related factors that might help predict treatment outcomes. The baseline characteristics that were most associated with likelihood of retention were the use of a slow PER titration schedule and a relatively low number of previous ASMs, but the low specificity of the resulting model (10.7%) compromises its validity. Multivariable regression analyses of effectiveness revealed that the baseline factors most associated with response to PER treatment and seizure freedom were the same; these being the presence of a genetic etiology, lower number of concomitant ASMs at baseline, absence of psychiatric comorbidity, lower number of previous ASMs, and higher age of onset of epilepsy. The high association of effectiveness with a genetic etiology may have resulted from the cohort containing a relatively high proportion of individuals with IGE, since idiopathic epilepsies have a relatively benign disease course and/or show a favorable response to ASM therapy [75]: 12.4% of the PERMIT population had epilepsy with a genetic etiology and 12.6% had only generalized seizures at baseline, broadly consistent with previous reports that IGE represents 15–20% of all epilepsies [76]. PER, which is already approved for the treatment of primary generalized tonic–clonic seizures [5,6,7], was demonstrated to be an appropriate and effective treatment for these PWE, who were potentially relatively refractory (median number of previous ASMs 4.0) and with a less benign disease course than the wider IGE population. The observed association between effectiveness and a relatively low number of previous and concomitant ASMs is perhaps to be expected, since these characteristics are associated with PWE who are relatively early in their disease course and/or responsive to ASM therapy. The association between effectiveness and a higher age of onset of epilepsy may support previous findings indicating that ASMs have superior effectiveness in older versus younger people with newly diagnosed epilepsy [77,78,79]. Previous studies of other ASMs have demonstrated that the presence of psychiatric comorbidity in epilepsy may be associated with a poor response to ASM therapy [80,81,82,83,84] and it is therefore perhaps unsurprising that absence of psychiatric comorbidity was associated with greater PER effectiveness in the current study. Epilepsy and psychiatric disorders, including depression, may share common pathogenic mechanisms that result in cortical hyperexcitability, worsening response to pharmacotherapy; such mechanisms include increased glutamatergic activity [82, 85, 86]. The presence of psychiatric comorbidity may additionally impact the effectiveness of ASM therapy by increasing the likelihood of treatment non-adherence and/or by increasing stress, which is known to be a common trigger for seizures in individuals with epilepsy [82, 87, 88].
PERMIT additionally provided insights into how PER was dosed and titrated in clinical practice. The median dose of PER was 2.0 mg/day at treatment initiation and 6.0 mg/day at the last visit, in line with treatment guidelines, which recommend initiating PER at 2.0 mg/day and up-titrating to a maintenance dose of 4–8 mg/day (maximum recommended dose, 12 mg/day) in adults [5]. The median number of concomitant ASMs remained unchanged during the study (2.0 at baseline and last visit) and the proportion of PWE treated with PER as monotherapy was low (5.6% at baseline; 4.1% at the last visit). A fast titration (2 mg/week) was used in approximately two-thirds of PWE (65.5%) and a slow titration (less than 2 mg/week) was used in the remaining third (34.5%). As previously mentioned, fast titration was associated with a significantly higher incidence of AEs and, in multivariable analysis, halved the likelihood of retention, in comparison with slow titration. Retention is a composite effectiveness outcome reflecting both efficacy and safety/tolerability, which is particularly relevant to clinical practice [89, 90]. It is therefore pertinent to speculate that the outcomes observed in PERMIT may perhaps have been improved if a slow titration rate had more commonly been used.
The main limitation of this investigation was that it was a retrospective pooled analysis of studies that were heterogeneous in terms of their objectives and information reported. It therefore employed a statistical analysis approach that attempted to report information in the most complete and harmonized way possible; however, data were not available for all PWE at all timepoints, across all endpoints and assessments. In addition, the majority of studies included in PERMIT were retrospective analyses of cases, which were necessarily uncontrolled and likely to have selection bias against patients with greater likelihood of suffering known adverse effects, thus altering the balance between side effects and effectiveness, in comparison with blinded studies. It is also important to highlight that seizure freedom was defined as no seizures since at least the prior visit (rather than no seizures since initiation of PER treatment), which could have been 3 or 6 months, depending on the timepoint concerned. The relatively low retention rate over the longer term (29.5%), in comparison with the retention rate at 12 months (64.2%), is likely to reflect the fact that patients are typically followed up until a study drug is withdrawn, leading to an underestimation of long-term retention; therefore, the retention time (mean, 18.7 months) might be a more relevant outcome measure over the longer term than the retention rate. Although individual subject data were previously reviewed by the investigators of the original studies, they were not reviewed systematically post hoc in the current study. Multivariable binary logistic regression analyses were exploratory in nature and the models generated were limited in terms of both sensitivity and specificity; the findings of these analyses should be therefore be interpreted with caution, but nevertheless provide additional insights supporting the study’s other findings.
In summary, the PERMIT study demonstrated that PER is effective and generally well tolerated when used to treat people with focal and/or generalized epilepsy in everyday clinical practice. Including over 5000 PWE, PERMIT is the largest pooled analysis of PER clinical practice data conducted to date, and provides reassuring evidence of PER’s safety/tolerability, with no new or unexpected side effects emerging with long-term use in the real-world setting. Over 50% of PWE responded to PER therapy, including those with status epilepticus, and almost a quarter of PWE were seizure free for at least 6 months after 12 months of treatment. These findings complement evidence from clinical trials, further supporting the use of PER for the treatment of both focal and generalized epilepsy.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Hanada T, Hashizume Y, Tokuhara N, Takenaka O, Kohmura N, Ogasawara A, Hatakeyama S, Ohgoh M, Ueno M, Nishizawa Y (2011) Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 52(7):1331–1340. https://doi.org/10.1111/j.1528-1167.2011.03109.x
Rogawski MA, Hanada T (2013) Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol Scand Suppl 197:19–24. https://doi.org/10.1111/ane.12100
Potschka H, Trinka E (2019) Perampanel: does it have broad-spectrum potential? Epilepsia 60(Suppl 1):22–36. https://doi.org/10.1111/epi.14456
Lattanzi S, Striano P (2019) The impact of perampanel and targeting AMPA transmission on anti-seizure drug discovery. Expert Opin Drug Discov 14(3):195–197. https://doi.org/10.1080/17460441.2019.1566318
European Medicines Agency (2021). Fycompa® (perampanel) summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/fycompa-epar-product-information_en.pdf. Accessed 22 July 2021
Food and Drug Administration (2021). Fycompa® (perampanel) prescribing information. Available at: https://www.fycompa.com/-/media/Files/Fycompa/Fycompa_Prescribing_Information.pdf. Accessed 22 July 2021
Therapeutic Goods Administration (2021). Fycompa® (perampanel) Prescribing information. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2014-PI-02011-1&d=202104061016933. Accessed 22 July 2021
French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, Kumar D, Rogawski MA (2012) Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology 79(6):589–596. https://doi.org/10.1212/WNL.0b013e3182635735
French JA, Krauss GL, Steinhoff BJ, Squillacote D, Yang H, Kumar D, Laurenza A (2013) Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia 54(1):117–125. https://doi.org/10.1111/j.1528-1167.2012.03638.x
Krauss GL, Serratosa JM, Villanueva V, Endziniene M, Hong Z, French J, Yang H, Squillacote D, Edwards HB, Zhu J, Laurenza A (2012) Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology 78(18):1408–1415. https://doi.org/10.1212/WNL.0b013e318254473a
Fogarasi A, Flamini R, Milh M, Phillips S, Yoshitomi S, Patten A, Takase T, Laurenza A, Ngo LY (2020) Open-label study to investigate the safety and efficacy of adjunctive perampanel in pediatric patients (4 to <12 years) with inadequately controlled focal seizures or generalized tonic-clonic seizures. Epilepsia 61(1):125–137. https://doi.org/10.1111/epi.16413
French JA, Krauss GL, Wechsler RT, Wang XF, DiVentura B, Brandt C, Trinka E, O’Brien TJ, Laurenza A, Patten A, Bibbiani F (2015) Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial. Neurology 85(11):950–957. https://doi.org/10.1212/WNL.0000000000001930
Tlusta E, Handoko KB, Majoie M, Egberts TC, Vlcek J, Heerdink ER (2008) Clinical relevance of patients with epilepsy included in clinical trials. Epilepsia 49(8):1479–1480. https://doi.org/10.1111/j.1528-1167.2008.01618_2.x
Arzimanoglou A, Ben-Menachem E, Cramer J, Glauser T, Seeruthun R, Harrison M (2010) The evolution of antiepileptic drug development and regulation. Epileptic Disord 12(1):3–15. https://doi.org/10.1684/epd.2010.0303
Steinhoff BJ, Staack AM, Hillenbrand BC (2017) Randomized controlled antiepileptic drug trials miss almost all patients with ongoing seizures. Epilepsy Behav 66:45–48. https://doi.org/10.1016/j.yebeh.2016.10.025
Rohracher A, Zimmermann G, Villanueva V, Garamendi I, Sander JW, Wehner T, Shankar R, Ben-Menachem E, Brodie MJ, Pensel MC, Di Gennaro G, Maurousset A, Strzelczyk A, Rheims S, Rácz A, Menzler K, Bertol-Alegre V, García-Morales I, López-González FJ, Toledo M, Carpenter KJ, Trinka E (2018) Perampanel in routine clinical use across Europe: pooled, multicenter, observational data. Epilepsia 59(9):1727–1739. https://doi.org/10.1111/epi.14520
Kim DW, Oh J (2018) One-year retention study of adjunctive perampanel treatment in epilepsy patients. Clin Neuropharmacol 41(1):10–13. https://doi.org/10.1097/WNF.0000000000000255
Abril Jaramillo J, Estévez María JC, Girón Úbeda JM, Vega López Ó, Calzado Rivas ME, Pérez Díaz H, García Martín G, Vila Herrero E, Chamorro-Muñoz M, Vázquez F, De la Fuente C, Redondo L, Peláez N, Santágueda P, Rodríguez Uranga JJ (2020) Effectiveness and safety of perampanel as early add-on treatment in patients with epilepsy and focal seizures in the routine clinical practice: Spain prospective study (PERADON). Epilepsy Behav 102:106655. https://doi.org/10.1016/j.yebeh.2019.106655
Santamarina E, Bertol V, Garayoa V, García-Gomara MJ, Garamendi-Ruiz I, Giner P, Aranzábal I, Piera A, Arcos C, Esteve P, Marinas A, García-Escrivá A, Viloria-Alebesque A, Loro FA, de Tienda AP, Olivan JA, Bonet M, Dávila-González P, Sivera R, Molins A, Sansa G, Roche JC, Martínez AB, Monteagudo S, Casadevall T (2020) Efficacy and tolerability of perampanel as a first add-on therapy with different anti-seizure drugs. Seizure 83:48–56. https://doi.org/10.1016/j.seizure.2020.09.026
Villanueva V, Montoya J, Castillo A, Mauri-Llerda JÁ, Giner P, López-González FJ, Piera A, Villanueva-Hernández P, Bertol V, Garcia-Escrivá A, Garcia-Peñas JJ, Garamendi I, Esteve-Belloch P, Baiges-Octavio JJ, Miró J, Falip M, Garcés M, Gómez A, Gil-López FJ, Carreño M, Rodriguez-Uranga JJ, Campos D, Bonet M, Querol R, Molins A, Tortosa D, Salas-Puig J (2018) Perampanel in routine clinical use in idiopathic generalized epilepsy: the 12-month GENERAL study. Epilepsia 59(9):1740–1752. https://doi.org/10.1111/epi.14522
Coppola A, Zarabla A, Maialetti A, Villani V, Koudriavtseva T, Russo E, Nozzolillo A, Sueri C, Belcastro V, Balestrini S, Ferlazzo E, Giannarelli D, Bilo L, Maschio M (2020) Perampanel confirms to be effective and well-tolerated as an add-on treatment in patients with brain tumor-related epilepsy (PERADET Study). Front Neurol 11:592. https://doi.org/10.3389/fneur.2020.00592
Toledo M, Gonzalez-Cuevas M, Miró-Lladó J, Molins-Albanell A, Falip M, Martinez AB, Fernandez S, Quintana M, Cambrodi R, Santamarina E, Salas-Puig J (2016) Sleep quality and daytime sleepiness in patients treated with adjunctive perampanel for focal seizures. Epilepsy Behav 63:57–62. https://doi.org/10.1016/j.yebeh.2016.08.004
Strzelczyk A, Knake S, Kälviäinen R, Santamarina E, Toledo M, Willig S, Rohracher A, Trinka E, Rosenow F (2019) Perampanel for treatment of status epilepticus in Austria, Finland, Germany, and Spain. Acta Neurol Scand 139(4):369–376. https://doi.org/10.1111/ane.13061
Gil-López FJ, Montoya J, Falip M, Aparicio J, López-González FJ, Toledano R, Gil-Nagel A, Molins A, García I, Serrano P, Domenech G, Torres F, Donaire A, Carreño M (2018) Retrospective study of perampanel efficacy and tolerability in myoclonic seizures. Acta Neurol Scand 138(2):122–129. https://doi.org/10.1111/ane.12931
Auvin S, Dozieres B, Ilea A, Delanoë C (2017) Use of perampanel in children and adolescents with Lennox-Gastaut Syndrome. Epilepsy Behav 74:59–63. https://doi.org/10.1016/j.yebeh.2017.05.036
Alsaadi T, Kassie SA, Servano R (2019) Efficacy and tolerability of perampanel in patients with genetic generalized epilepsy (GGE): a retrospective, single-center study from the United Arab Emirates (UAE). Epilepsy Behav Rep 12:100330. https://doi.org/10.1016/j.ebr.2019.100330
Izumoto S, Miyauchi M, Tasaki T, Okuda T, Nakagawa N, Nakano N, Kato A, Fujita M (2018) Seizures and tumor progression in glioma patients with uncontrollable epilepsy treated with perampanel. Anticancer Res 38(7):4361–4366. https://doi.org/10.21873/anticanres.12737
Deleo F, Quintas R, Turner K, Didato G, Zambrelli E, Pappalardo I, Chiesa V, Pastori C, de Curtis M, Canevini MP, Villani F (2019) The impact of perampanel treatment on quality of life and psychiatric symptoms in patients with drug-resistant focal epilepsy: an observational study in Italy. Epilepsy Behav 99:106391. https://doi.org/10.1016/j.yebeh.2019.06.034
Maschio M, Pauletto G, Zarabla A, Maialetti A, Ius T, Villani V, Fabi A, Koudriavtseva T, Giannarelli D (2019) Perampanel in patients with brain tumor-related epilepsy in real-life clinical practice: a retrospective analysis. Int J Neurosci 129(6):593–597. https://doi.org/10.1080/00207454.2018.1555160
Steinhoff BJ, Hübers E, Kurth C, Jürges Kehl-Kork U (2019) Plasma concentration and clinical effects of perampanel—the Kork experience. Seizure 67:18–22. https://doi.org/10.1016/j.seizure.2019.02.022
Rea R, Traini E, Renna R, Pagliuca F, Pezzella M, Pagliuca M (2019) Efficacy and impact on cognitive functions and quality of life of perampanel as first add-on therapy in patients with epilepsy: a retrospective study. Epilepsy Behav 98(Pt A):139–144. https://doi.org/10.1016/j.yebeh.2019.07.005
Toledano Delgado R, García-Morales I, Parejo-Carbonell B, Jiménez-Huete A, Herrera-Ramirez D, González-Hernández A, Ayuga Loro F, Santamarina E, Toledo M, Ojeda J, Poza JJ, Molins A, Giner P, Estévez María JC, Castro-Vilanova MD, Zurita J, Saiz-Diaz RA, Gómez-Ibañez A, Rodriguez-Uranga J, Gil-Nagel A, Campos D, Sánchez-Larsen Á, Aguilar-Amat Prior MJ, Mauri Llerda JA, Huertas González N, García-Barragán N (2020) Effectiveness and safety of perampanel monotherapy for focal and generalized tonic-clonic seizures: experience from a national multicenter registry. Epilepsia 61(6):1109–1119. https://doi.org/10.1111/epi.16548
Moraes JS, Hepworth G, Ignatiadis S, Dharan A, Carne R, Seneviratne U, Cook MJ, D’Souza WJ (2020) Improved irritability, mood, and quality of life following introduction of perampanel as late adjunctive treatment for epilepsy. Epilepsy Behav 104(Pt A):106883. https://doi.org/10.1016/j.yebeh.2019.106883
Chiang HI, Lim SN, Hsieh HY, Cheng MY, Chang CW, Johnny Tseng WE, Li HT, Lin CY, Wu T (2017) Preliminary Asian experience of using perampanel in clinical practice. Biomed J 40(6):347–354. https://doi.org/10.1016/j.bj.2017.09.003
Datta AN, Xu Q, Sachedina S, Boelman C, Huh L, Connolly MB (2017) Clinical experience with perampanel for refractory pediatric epilepsy in one Canadian center. J Child Neurol 32(9):834–839. https://doi.org/10.1177/0883073817709195
Coyle H, Clough P, Cooper P, Mohanraj R (2014) Clinical experience with perampanel: focus on psychiatric adverse effects. Epilepsy Behav 41:193–196. https://doi.org/10.1016/j.yebeh.2014.09.072
Ho CJ, Lin CH, Lu YT, Shih FY, Hsu CW, Tsai WC, Tsai MH (2019) Perampanel treatment for refractory status epilepticus in a neurological intensive care unit. Neurocrit Care 31(1):24–29. https://doi.org/10.1007/s12028-019-00704-9
De Liso P, Vigevano F, Specchio N, De Palma L, Bonanni P, Osanni E, Coppola G, Parisi P, Grosso S, Verrotti A, Spalice A, Nicita F, Zamponi N, Siliquini S, Giordano L, Martelli P, Guerrini R, Rosati A, Ilvento L, Belcastro V, Striano P, Vari MS, Capovilla G, Beccaria F, Bruni O, Luchetti A, Gobbi G, Russo A, Pruna D, Tozzi AE, Cusmai R (2016) Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies—an Italian observational multicenter study. Epilepsy Res 127:93–100. https://doi.org/10.1016/j.eplepsyres.2016.08.021
Liguori C, Izzi F, Manfredi N, D’Elia A, Mari L, Mercuri NB, Fabio P (2018) Efficacy and tolerability of perampanel and levetiracetam as first add-on therapy in patients with epilepsy: a retrospective single center study. Epilepsy Behav 80:173–176. https://doi.org/10.1016/j.yebeh.2018.01.001
Liguori C, Turner K, Izzi F, Assogna M, Canevini MP, Mercuri NB, Placidi F (2019) Preliminary evidence about irritability in patients with epilepsy treated by perampanel as first add-on therapy compared to levetiracetam and valproic acid. CNS Neurosci Ther 25(5):632–637. https://doi.org/10.1111/cns.13098
Takahashi S, Shimizu K, Inaji M, Hashimoto S, Yamamoto S, Maehara T (2019) Effectiveness of perampanel as a first add-on antiepileptic drug for the treatment of partial epilepsy. Epilepsy Behav 100(Pt A):106492. https://doi.org/10.1016/j.yebeh.2019.106492
Morano A, Fattouch J, Albini M, Casciato S, Fanella M, Basili LM, Viganò A, Manfredi M, Giallonardo AT, Di Bonaventura C (2018) Perampanel as adjunctive therapy in highly refractory epilepsies: real-world data from an Italian tertiary care epilepsy centre. J Neurol Sci 390:67–74. https://doi.org/10.1016/j.jns.2018.04.017
Goji H, Kanemoto K (2019) The effect of perampanel on aggression and depression in patients with epilepsy: a short-term prospective study. Seizure 67:1–4. https://doi.org/10.1016/j.seizure.2019.02.009
Rocamora R, Álvarez I, Chavarría B, Principe A (2020) Perampanel effect on sleep architecture in patients with epilepsy. Seizure 76:137–142. https://doi.org/10.1016/j.seizure.2020.01.021
Gil-Nagel A, Burd S, Toledo M, Sander JW, Lebedeva A, Patten A, Laurenza A, Study 504 investigator group (2018) A retrospective, multicentre study of perampanel given as monotherapy in routine clinical care in people with epilepsy. Seizure 54:61–66. https://doi.org/10.1016/j.seizure.2017.10.015
Vlasov PN, Karlov VA, Zhidkova IA, Dmitrenko DV, Rudakova IG, Danilova TV, Kalinin VA, Grebenyuk OV, Gertsen AP, Zhuravlev YS, Karas AY, Paramonova EN, Ponomareva IV, Miguskina OI, Sobyanina NA, Sukhova DV, Salomatin YV, Ertakhova ML, Goguadze TM, Shamray AP (2020) A Russian retrospective multicenter open-label observational study based on medical documentation on the use of perampanel in everyday clinical practice. Neurol Neuropsychiatry Psychosomatics 12(3):47–55. https://doi.org/10.14412/2074-2711-2020-3-47-55
Teijeira Azcona AL, Ayuga Loro F, Cabeza Alvarez CI, Almansa Castillo R (2018) Effectiveness of perampanel as first add-on and monotherapy in clinical practice: a single-center observational study in Spain. Presented at American Epilepsy Society Annual Meeting 2018. https://cms.aesnet.org/abstractslisting/effectiveness-of-perampanel-as-first-add-on-and-monotherapy-in-clinical-practice--a-single-center-observational-study-in-spain. Accessed 22 July 2021
Ron AG, Arlandis MT, Losada R, Soto V, Santos MD, Fernandez PR (2018) Neurocognitive safety of perampanel as adjunctive therapy for refractory epilepsy in childhood and its relation with sleep. Epilepsia 59(Suppl 3):S193-S194 (abstract p421). https://doi.org/10.1111/epi.14612
Carreño M, Gil F, Conde E, Manzanares I (2018) Efficacy and tolerability of perampanel to treat drug resistant sleep seizures. Epilepsia 59(Suppl. 3):S275 (abstract p604). https://doi.org/10.1111/epi.14612
Yamamoto T (2018) Treatment of epilepsy by perampanel at acute care hospitals through a comprehensive epilepsy center and its effectiveness of early add-on. Presented at American Epilepsy Society Annual Meeting 2018. https://cms.aesnet.org/abstractslisting/treatment-of-epilepsy-by-perampanel-at-acute-care-hospitals-through-a-comprehensive-epilepsy-center-and-its-effectiveness-of-early-add-on. Accessed 22 July 2021
Osorio XR, López-González J, Lema-Facal T, Rubio-Nazábal E, Castro-Vilanova MD, Pato-Pato A, Abella-Corral J, Corredera E, López-Ferreiro A, Puy-Núñez A (2018) Perampanel efficacy and safety as early add-on treatment for focal seizures: Pereagal study. Epilepsia 59(Suppl. 3):S177 (abstract p382). https://doi.org/10.1111/epi.14612
Odintsova G (2018) Influence of perampanel on behavior and cognition. Epilepsia 59(Suppl. 3):S177 (abstract p383). https://doi.org/10.1111/epi.14612
Matricardi S, Siliquini S, Lattanzi S, Cagnetti C, Deleo F, Stabile A, Ragona F, Freri E, Cesaroni E, Anibaldi G, Foschi N, Villani F, Granata T, Zamponi N (2018) Retention rate and outcome-related factors of perampanel: a real-life population study. Epilepsia 59(Suppl. 3):S78 (abstract p158). https://doi.org/10.1111/epi.14612
Chinvarun Y (2019) Perampanel as monotherapy for new onset focal seizures: real world experience. Presented at American Epilepsy Society Annual Meeting 2019. https://cms.aesnet.org/abstractslisting/perampanel-as-monotherapy-for-new-onset-focal-seizures--real-world-experienced. Accessed 22 July 2021
Kristensen T (2016) Retrospective study with perampanel in the Fjord district in Norway from 2013 up to February 2016 Patients diagnosed with POS and PGTC. Epilepsia 57(Suppl. 2):188 (abstract P618). https://doi.org/10.1111/epi.13609
Bonanni P, Negrin S, Danieli A, Osanni E (2017) Effectiveness and tolerability of perampanel in patients with intellectual disability and refractory epilepsy. Epilepsia 58(Suppl. 5):S110-S111 (abstract p0597). https://doi.org/10.1111/epi.13944
Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Roulet Perez E, Scheffer IE, Zuberi SM (2017) Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia 58(4):522–530. https://doi.org/10.1111/epi.13670
Villanueva V, Garcés M, López-González FJ, Rodriguez-Osorio X, Toledo M, Salas-Puig J, González-Cuevas M, Campos D, Serratosa JM, González-Giráldez B, Mauri JA, Camacho JL, Suller A, Carreño M, Gómez JB, Montoya J, Rodríguez-Uranga J, Saiz-Diaz R, González-de la Aleja J, Castillo A, López-Trigo J, Poza JJ, Flores J, Querol R, Ojeda J, Giner P, Molins A, Esteve P, Baiges JJ (2016) Safety, efficacy and outcome-related factors of perampanel over 12 months in a real-world setting: the FYDATA study. Epilepsy Res 126:201–210. https://doi.org/10.1016/j.eplepsyres.2016.08.001
Shah E, Reuber M, Goulding P, Flynn C, Delanty N, Kemp S (2016) Clinical experience with adjunctive perampanel in adult patients with uncontrolled epilepsy: a UK and Ireland multicentre study. Seizure 34:1–5. https://doi.org/10.1016/j.seizure.2015.10.017
Hasegawa N, Tohyama J (2021) Positive and negative effects of perampanel treatment on psychiatric and behavioral symptoms in adult patients with epilepsy. Epilepsy Behav 117:107515. https://doi.org/10.1016/j.yebeh.2020.107515
Chung S, Williams B, Dobrinsky C, Patten A, Yang H, Laurenza A (2017) Perampanel with concomitant levetiracetam and topiramate: post hoc analysis of adverse events related to hostility and aggression. Epilepsy Behav 75:79–85. https://doi.org/10.1016/j.yebeh.2017.06.038
Ettinger AB, LoPresti A, Yang H, Williams B, Zhou S, Fain R, Laurenza A (2015) Psychiatric and behavioral adverse events in randomized clinical studies of the noncompetitive AMPA receptor antagonist perampanel. Epilepsia 56(8):1252–1263. https://doi.org/10.1111/epi.13054
Leitinger M, Trinka E, Giovannini G, Zimmermann G, Florea C, Rohracher A, Kalss G, Neuray C, Kreidenhuber R, Höfler J, Kuchukhidze G, Granbichler C, Dobesberger J, Novak HF, Pilz G, Meletti S, Siebert U (2019) Epidemiology of status epilepticus in adults: a population-based study on incidence, causes, and outcomes. Epilepsia 60(1):53–62. https://doi.org/10.1111/epi.14607
Trinka E, Höfler J, Zerbs A (2012) Causes of status epilepticus. Epilepsia 53(Suppl. 4):127–138. https://doi.org/10.1111/j.1528-1167.2012.03622.x
Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, Shorvon S, Lowenstein DH (2015) A definition and classification of status epilepticus—report of the ILAE task force on classification of status epilepticus. Epilepsia 56(10):1515–1523. https://doi.org/10.1111/epi.13121
Kim D, Kim JM, Cho YW, Yang KI, Kim DW, Lee ST, No YJ, Seo JG, Byun JI, Kang KW, Kim KT, Drug Committee of Korean Epilepsy Society (2021) Antiepileptic drug therapy for status epilepticus. J Clin Neurol 17(1):11–19. https://doi.org/10.3988/jcn.2021.17.1.11
Ferlisi M, Hocker S, Trinka E, Shorvon S, International Steering Committee of the StEp Audit (2018) Etiologies and characteristics of refractory status epilepticus cases in different areas of the world: results from a global audit. Epilepsia 59(Suppl. 2):100–107. https://doi.org/10.1111/epi.14496
Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, Bare M, Bleck T, Dodson WE, Garrity L, Jagoda A, Lowenstein D, Pellock J, Riviello J, Sloan E, Treiman DM (2016) Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr 16(1):48–61. https://doi.org/10.5698/1535-7597-16.1.48
Trinka E, Höfler J, Leitinger M, Rohracher A, Kalss G, Brigo F (2016) Pharmacologic treatment of status epilepticus. Expert Opin Pharmacother 17(4):513–534. https://doi.org/10.1517/14656566.2016.1127354
Brigo F, Del Giovane C, Nardone R, Trinka E, Lattanzi S (2019) Intravenous antiepileptic drugs in adults with benzodiazepine-resistant convulsive status epilepticus: a systematic review and network meta-analysis. Epilepsy Behav 101(Pt B):106466. https://doi.org/10.1016/j.yebeh.2019.106466
Trinka E, Höfler J, Leitinger M, Brigo F (2015) Pharmacotherapy for status epilepticus. Drugs 75(13):1499–1521. https://doi.org/10.1007/s40265-015-0454-2
Brigo F, Lattanzi S, Rohracher A, Russo E, Meletti S, Grillo E, Trinka E (2018) Perampanel in the treatment of status epilepticus: a systematic review of the literature. Epilepsy Behav 86:179–186. https://doi.org/10.1016/j.yebeh.2018.07.004
Alsherbini K, Abhi Pandhi F, Goyanes J, Deep A, Jones GM (2020) A retrospective, observational study of perampanel in refractory and super-refractory status epilepticus. J Neurol Sci 419:117214. https://doi.org/10.1016/j.jns.2020.117214
Lim SN, Wu T, Tseng WJ, Chiang HI, Cheng MY, Lin WR, Lin CN (2021) Efficacy and safety of perampanel in refractory and super-refractory status epilepticus: cohort study of 81 patients and literature review. J Neurol 21. [online ahead of print] https://doi.org/10.1007/s00415-021-10506-9
Perucca E (2001) The management of refractory idiopathic epilepsies. Epilepsia 42(Suppl. 3):31–35. https://doi.org/10.1046/j.1528-1157.2001.042suppl.3031.x
Jallon P, Latour P (2005) Epidemiology of idiopathic generalized epilepsies. Epilepsia 46(Suppl. 9):10–14. https://doi.org/10.1111/j.1528-1167.2005.00309.x
Stephen LJ, Kelly K, Mohanraj R, Brodie MJ (2006) Pharmacological outcomes in older people with newly diagnosed epilepsy. Epilepsy Behav 8(2):434–437. https://doi.org/10.1016/j.yebeh.2005.11.007
Brodie MJ, Stephen LJ (2007) Outcomes in elderly patients with newly diagnosed and treated epilepsy. Int Rev Neurobiol 81:253–263. https://doi.org/10.1016/S0074-7742(06)81016-0
Lawthom C, Bermejo P, Campos D, McMurray R, Villanueva V (2019) Effectiveness and safety/tolerability of eslicarbazepine acetate in epilepsy patients aged ≥ 60 versus < 60 years: a subanalysis from the Euro-Esli study. Neurol Ther 8(2):491–504. https://doi.org/10.1007/s40120-019-0137-0
Petrovski S, Szoeke CE, Jones NC, Salzberg MR, Sheffield LJ, Huggins RM, O’Brien TJ (2010) Neuropsychiatric symptomatology predicts seizure recurrence in newly treated patients. Neurology 75(11):1015–1021. https://doi.org/10.1212/WNL.0b013e3181f25b16
Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ (2007) Predictors of pharmacoresistant epilepsy. Epilepsy Res 75(2–3):192–196. https://doi.org/10.1016/j.eplepsyres.2007.06.003
Kanner AM (2017) Psychiatric comorbidities in new onset epilepsy: should they be always investigated? Seizure 49:79–82. https://doi.org/10.1016/j.seizure.2017.04.007
Chen B, Choi H, Hirsch LJ, Legge A, Buchsbaum R, Detyniecki K (2018) Cross-sensitivity of psychiatric and behavioral side effects with antiepileptic drug use. Seizure 62:38–42. https://doi.org/10.1016/j.seizure.2018.09.014
Schmitz B, Dimova S, Zhang Y, Chellun D, De Backer M, Gasalla T (2020) Tolerability and efficacy of lacosamide and controlled-release carbamazepine monotherapy in patients with newly diagnosed epilepsy and concomitant psychiatric conditions: post hoc analysis of a prospective, randomized, double-blind trial. Epilepsy Res 159:106220. https://doi.org/10.1016/j.eplepsyres.2019.106220
Kanner AM (2012) Can neurobiological pathogenic mechanisms of depression facilitate the development of seizure disorders? Lancet Neurol 11(12):1093–1102. https://doi.org/10.1016/S1474-4422(12)70201-6
Kanner AM, Mazarati A, Koepp M (2014) Biomarkers of epileptogenesis: psychiatric comorbidities (?). Neurotherapeutics 11(2):358–372. https://doi.org/10.1007/s13311-014-0271-4
Trinka E, Kienpointner G, Unterberger I, Luef G, Bauer G, Doering LB, Doering S (2006) Psychiatric comorbidity in juvenile myoclonic epilepsy. Epilepsia 47(12):2086–2091. https://doi.org/10.1111/j.1528-1167.2006.00828.x
McKee HR, Privitera MD (2017) Stress as a seizure precipitant: Identification, associated factors, and treatment options. Seizure 44:21–26. https://doi.org/10.1016/j.seizure.2016.12.009
International League Against Epilepsy (1998) Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia 39(7):799–803. https://doi.org/10.1111/j.1528-1157.1998.tb01167.x
Kessler SK, McCarthy A, Cnaan A, Dlugos DJ (2015) Retention rates of rufinamide in pediatric epilepsy patients with and without Lennox-Gastaut Syndrome. Epilepsy Res 112:18–26. https://doi.org/10.1016/j.eplepsyres.2015.02.003
Acknowledgements
The following clinicians involved in studies included in PERMIT: J Abril Jaramillo, T Alsaadi, S Auvin, E Ben-Menachem, V Bertol-Alegre, P Bonanni, MJ Brodie, KJ Carpenter, R Carne, M Carreño, HI Chiang, Y Chinvarun, A Coppola, M Cook, H Coyle, AN Datta, P De Liso, F Deleo, M Valente Fernandes, G Di Gennaro, J Frasca, I Garamendi, I García-Morales, FJ Gil-López, A Gil-Nagel, CJ Ho, S Izumoto, J Jacobs-leVan, M Kiley, T Kristensen, N Lawn, FJ López-González, A Suller Marti, M Maschio, S Matricardi, A Maurousset, K Menzler, JS Moraes, A Morano, L Morillo, A Nikpour, G Odintsova, XR Osorio, MC Pensel, A Rácz, R Rea, S Rheims, R Rocamora, A Rohracher, AG Ron, JW Sander, R Shankar, U Seneviratne, A Strzelczyk, S Takahashi, AL Teijeira Azcona, R Toledano Delgado, M Toledo, T Wehner, E Whitham, T Yamamoto and G Zimmerman. This study was funded by Eisai Ltd. Editorial assistance was provided by John Scopes of mXm Medical Communications and funded by Eisai Ltd.
Funding
This study was funded by Eisai Ltd. Editorial assistance in the preparation of this manuscript was funded by Eisai Ltd.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
Vicente Villanueva has received honoraria and/or research funds from Angelini, Arvelle, Bial, Eisai, Esteve, GW Pharma, NewBridge, Novartis, UCB Pharma and Zogenix. Wendyl D’Souza’s salary is part-funded by The University of Melbourne. He has received travel, investigator-initiated, scientific advisory board and speaker honoraria from UCB Pharma Australia & Global; investigator-initiated, scientific advisory board, travel and speaker honoraria from Eisai Australia & Global; advisory board honoraria from Liva Nova and Tilray; educational grants from Novartis Pharmaceuticals, Pfizer Pharmaceuticals and Sanofi-Synthelabo; educational, travel and fellowship grants from GSK Neurology Australia; and honoraria from SciGen Pharmaceuticals. He has an equity interest in the device company EpiMinder and health software company KeyLead Health. Hiroko Goji has no conflicts of interest. Dong Wook Kim has no conflicts of interest. Claudio Liguori has no conflicts of interest. Rob McMurray is an employee of Eisai Europe Ltd. Imad Najm has received honoraria from Eisai. Estevo Santamarina has received honoraria and/or research funds from Bial, Eisai, Esteve, GW Pharma and UCB Pharma. Bernhard J. Steinhoff has received consultation fees and/or honoraria from Arvelle, B. Braun Melsungen, Desitin, Eisai, GW Pharma, Neuraxpharm, Precisis, UCB Pharma, and Zogenix. Pavel Vlasov has received consultation fees and/or honoraria from Alkaloid, Eisai, Novartis, Sanofi, Teva and UCB Pharma. Tony Wu has no conflicts of interest. Eugen Trinka has received consultation fees and/or speakers honoraria from Angelini, Argenx, Arvelle, Bial, Biogen-Idec, Boehringer Ingelheim, Eisai, Epilog, EVER Pharma, Genzyme, GL Pharma, GW Pharmaceuticals, Hikma, LivaNova, Marinus, Medtronic, NewBridge, Novartis, Sanofi, and UCB Pharma.
Ethics approval
All human studies outlined in this article were approved by the appropriate ethics committees and have, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
All persons gave their informed consent prior to their inclusion in the studies, according to the protocol.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Villanueva, V., D’Souza, W., Goji, H. et al. PERMIT study: a global pooled analysis study of the effectiveness and tolerability of perampanel in routine clinical practice. J Neurol 269, 1957–1977 (2022). https://doi.org/10.1007/s00415-021-10751-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10751-y