Abstract
Background
MRI atrophy predicts cognitive status in AD. However, this relationship has not been investigated in early-onset AD (EOAD, < 65 years) patients with a biomarker-based diagnosis.
Methods
Forty eight EOAD (MMSE ≥ 15; A + T + N +) and forty two age-matched healthy controls (HC; A − T − N −) from a prospective cohort underwent full neuropsychological assessment, 3T-MRI scan and lumbar puncture at baseline. Participants repeated the cognitive assessment annually. We used linear mixed models to investigate whether baseline cortical thickness (CTh) or subcortical volume predicts two-year cognitive outcomes in the EOAD group.
Results
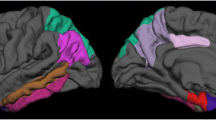
In EOAD, hemispheric CTh and ventricular volume at baseline were associated with global cognition, language and attentional/executive functioning 2 years later (p < 0.0028). Regional CTh was related to most cognitive outcomes (p < 0.0028), except verbal/visual memory subtests. Amygdalar volume was associated with letter fluency test (p < 0.0028). Hippocampal volume did not show significant associations.
Conclusion
Baseline hemispheric/regional CTh, ventricular and amygdalar volume, but not the hippocampus, predict two-year cognitive outcomes in EOAD.




Similar content being viewed by others
Data availability
The summary tables that support the findings of this study are available on request from the corresponding author, AL.
Code availability
Code used in this study is available on request from the corresponding author, AL.
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid-β
- bankssts:
-
Banks of the superior temporal sulcus
- BDAE:
-
The Boston Diagnostic Aphasia Examination
- BNT:
-
Boston Naming Test
- CERAD:
-
Consortium to Establish a Registry for Alzheimer’s disease
- CSF:
-
Cerebrospinal fluid
- CTh:
-
Cortical thickness
- DFR:
-
Delayed free recall
- Digits-B:
-
Digits span backwards
- Digits-F:
-
Digits span forwards
- DTR:
-
Delayed total recall
- EOAD:
-
Early-onset AD
- LFT:
-
Letter fluency test
- FCSRT:
-
Free and Cued Selective Reminding Test
- FDG-PET:
-
Fluorodeoxyglucose positron emission tomography
- FL:
-
Free learning
- GC:
-
Global composite
- HC:
-
Healthy controls
- HCB:
-
Hospital Clínic de Barcelona
- LM:
-
Linear model
- LME:
-
Linear mixed-effects model
- LOAD:
-
Late-onset AD
- MMSE:
-
Mini-Mental State Examination
- MRI:
-
Magnetic resonance imaging
- MTL:
-
Medial temporal lobe
- NIA-AA:
-
National Institute on Aging-Alzheimer’s Association
- SF:
-
Semantic fluency test
- TL:
-
Total learning
- TMT-A:
-
Trail Making Test-A
- VOSP:
-
Visual object and space perception battery
- WAB:
-
Western Aphasia Battery
- WAIS:
-
Wechsler Adult Intelligence Scale
- YOE:
-
Years of education
References
Jack CR, Knopman DS, Jagust WJ et al (2013) Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12:207–216. https://doi.org/10.1016/S1474-4422(12)70291-0
Murray ME, Graff-Radford NR, Ross OA et al (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796. https://doi.org/10.1016/S1474-4422(11)70156-9
Mendez MF (2012) Early-onset Alzheimer’s disease: nonamnestic subtypes and type 2 AD. Arch Med Res 43:677–685. https://doi.org/10.1016/j.arcmed.2012.11.009
Ossenkoppele R, Cohn-Sheehy BI, La Joie R et al (2015) Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Hum Brain Mapp 36:4421–4437. https://doi.org/10.1002/hbm.22927
Aziz AL, Giusiano B, Joubert S et al (2017) Difference in imaging biomarkers of neurodegeneration between early and late-onset amnestic Alzheimer’s disease. Neurobiol Aging 54:22–30. https://doi.org/10.1016/j.neurobiolaging.2017.02.010
Harper L, Bouwman F, Burton EJ et al (2017) Patterns of atrophy in pathologically confirmed dementias: a voxelwise analysis. J Neurol Neurosurg Psychiatry 88:908–916. https://doi.org/10.1136/jnnp-2016-314978
Falgàs N, Sánchez-Valle R, Bargalló N et al (2019) Hippocampal atrophy has limited usefulness as a diagnostic biomarker on the early onset Alzheimer’s disease patients: a comparison between visual and quantitative assessment. NeuroImage Clin 23:101927. https://doi.org/10.1016/j.nicl.2019.101927
Koedam ELGE, Lauffer V, Van Der Vlies AE et al (2010) Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimer’s Dis 19:1401–1408. https://doi.org/10.3233/JAD-2010-1337
Balasa M, Gelpi E, Antonell A et al (2011) Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology 76:1720–1725. https://doi.org/10.1212/WNL.0b013e31821a44dd
Jack CR, Bennett DA, Blennow K et al (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement 14:535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Schuff N, Woerner N, Boreta L et al (2009) MRI of hippocampal volume loss in early Alzheimers disease in relation to ApoE genotype and biomarkers. Brain 132:1067–1077. https://doi.org/10.1093/brain/awp007
Van Rossum IA, Vos SJB, Burns L et al (2012) Injury markers predict time to dementia in subjects with MCI and amyloid pathology. Neurology 79:1809–1816. https://doi.org/10.1212/WNL.0b013e3182704056
Marizzoni M, Ferrari C, Jovicich J et al (2019) Predicting and tracking short term disease progression in amnestic mild cognitive impairment patients with prodromal Alzheimer’s disease: structural brain biomarkers. J Alzheimer’s Dis 69:3–14. https://doi.org/10.3233/JAD-180152
Tabatabaei-Jafari H, Shaw ME, Walsh E, Cherbuin N (2019) Regional brain atrophy predicts time to conversion to Alzheimer’s disease, dependent on baseline volume. Neurobiol Aging 83:86–94. https://doi.org/10.1016/j.neurobiolaging.2019.08.033
Phillips ML, Stage EC, Lane KA et al (2019) Neurodegenerative patterns of cognitive clusters of early-onset Alzheimer’s disease subjects: evidence for disease heterogeneity. Dement Geriatr Cogn Disord 46202:1–12. https://doi.org/10.1159/000504341
Van Der Vlies AE, Staekenborg SS, Admiraal-Behloul F et al (2013) Associations between magnetic resonance imaging measures and neuropsychological impairment in early and late onset Alzheimer’s disease. J Alzheimer’s Dis 35:169–178. https://doi.org/10.3233/JAD-121291
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7:270–279. https://doi.org/10.1016/j.jalz.2011.03.008
Antonell A, Tort-Merino A, Ríos J et al (2020) Synaptic, axonal damage and inflammatory cerebrospinal fluid biomarkers in neurodegenerative dementias. Alzheimer’s Dement 16:262–272. https://doi.org/10.1016/j.jalz.2019.09.001
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. https://doi.org/10.1016/0022-3956(75)90026-6
Grober E, Buschke H, Korey SR (1987) Genuine memory deficits in dementia. Dev Neuropsychol 3:13–36. https://doi.org/10.1080/87565648709540361
Valls Pedret C, Olives Cladera J, Bosch Capdevila B et al (2011) Test de paisajes para la valoración de la memoria visual en la enfermedad de Alzheimer. Rev Neurol 53:1. https://doi.org/10.33588/rn.5301.2011238
Kaplan E, Goodglass HWS (1983) The Boston Naming Test. Philadelphia Lea Febiger
Roth C (2011) Boston Diagnostic Aphasia Examination. In: Jeffrey SK, John D, Bruce C (eds) Encyclopedia of Clinical Neuropsychology. Springer, New York
Goodglass H, Kaplan E, Barresi B (2001) The Assessment of Aphasia and Related Disorders. Lippincott Williams & Wilkins
Kertesz A (2007) Western Aphasia Battery: Revised. Pearson
Morris JC, Heyman A, Mohs RC et al (1989) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. https://doi.org/10.1212/wnl.39.9.1159
Warrington EK, James M (1991) The visual object and space perception battery
Reitan RM, Wolfson D (1985) The Halstead-Reitan Neuropsychological Test Battery: theory and clinical interpretation. Neuropsychology Press, USA
Newcombe F (1969) Missile Wounds of the Brain: a Study of Psychological Deficits. Oxford U.P.
Wechsler D (2008) WAIS-IV : Wechsler adult intelligence scale. TX Psychol Corp, San Antonio
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. https://doi.org/10.1073/pnas.200033797
Fischl B, van der Kouwe A, Destrieux C et al (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. https://doi.org/10.1093/cercor/bhg087
Desikan RS, Ségonne F, Fischl B et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021
Seidman LJ, Faraone SV, Goldstein JM et al (1997) Reduced subcortical brain volumes in nonpsychotic siblings of schizophrenic patients: a pilot magnetic resonance imaging study. Am J Med Genet Neuropsychiatr Genet 74:507–514. https://doi.org/10.1002/(SICI)1096-8628(19970919)74:5%3c507::AID-AJMG11%3e3.0.CO;2-G
Palasí A, Gutiérrez-Iglesias B, Alegret M et al (2015) Differentiated clinical presentation of early and late-onset Alzheimer’s disease: is 65 years of age providing a reliable threshold? J Neurol 262:1238–1246. https://doi.org/10.1007/s00415-015-7698-3
Smits LL, Pijnenburg YAL, Koedam ELGE et al (2012) Early onset Alzheimer’s disease is associated with a distinct neuropsychological profile. J Alzheimer’s Dis 30:101–108. https://doi.org/10.3233/JAD-2012-111934
Wattmo C, Wallin ÅK (2017) Early- versus late-onset Alzheimer disease: long-term functional outcomes, nursing home placement, and risk factors for rate of progression. Dement Geriatr Cogn Dis Extra 7:172–187. https://doi.org/10.1159/000455943
Pontecorvo MJ, Devous MD, Navitsky M et al (2017) Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain 140:748–763. https://doi.org/10.1093/brain/aww334
Koychev I, Gunn RN, Firouzian A et al (2017) PET tau and amyloid-β burden in mild Alzheimer’s disease: divergent relationship with age, cognition, and cerebrospinal fluid biomarkers. J Alzheimer’s Dis 60:283–293. https://doi.org/10.3233/JAD-170129
Quenon L, Dricot L, Woodard JL et al (2016) Prediction of free and cued selective reminding test performance using volumetric and amyloid-based biomarkers of Alzheimer’s disease. J Int Neuropsychol Soc 22:991–1004. https://doi.org/10.1017/S1355617716000813
Ossenkoppele R, Smith R, Ohlsson T et al (2019) Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology 92:e601–e612. https://doi.org/10.1212/WNL.0000000000006875
Whitwell JL, Dickson DW, Murray ME et al (2012) Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol 11:868–877. https://doi.org/10.1016/S1474-4422(12)70200-4
Risacher SL, Anderson WH, Charil A et al (2017) Alzheimer disease brain atrophy subtypes are associated with cognition and rate of decline. Neurology 89:2176–2186. https://doi.org/10.1212/WNL.0000000000004670
Park KH, Noh Y, Choi EJ et al (2017) Functional connectivity of the hippocampus in early- and vs. late-onset alzheimer’s disease. J Clin Neurol 13:387–393. https://doi.org/10.3988/jcn.2017.13.4.387
Dickerson BC, Brickhouse M, McGinnis S, Wolk DA (2017) Alzheimer’s disease: The influence of age on clinical heterogeneity through the human brain connectome. Alzheimer’s Dement Diagnosis. Assess Dis Monit 6:122–135. https://doi.org/10.1016/j.dadm.2016.12.007
Acknowledgements
The authors thank patients, their relatives and healthy controls for their participation in the research. We acknowledge support for the project provided by Spanish Ministry of Science and Innovation-Instituto de Salud Carlos III, Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, “Una manera de hacer Europa”, CERCA Programme/Generalitat de Catalunya and Departament de Salut—Generalitat de Catalunya (PERIS 2016-2020). We also are indebted to the Magnetic Resonance Image core facility of the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) for the technical help.
Funding
This work was supported by Spanish Ministry of Science and Innovation-Instituto de Salud Carlos III and Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, “Una manera de hacer Europa” [PI19/00449 to Dr. Lladó (AL)] and CERCA Programme/Generalitat de Catalunya. AL also received funding from Departament de Salut—Generalitat de Catalunya (PERIS 2016–2020 SLT008/18/00061). Roser Sala-Llonch received funding from the Biomedical Imaging Group, Biomedical Research Networking Center in Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN), Barcelona, Spain. Nuria Guillén received funding from a PFIS grant (FI20/00076). Oscar Ramos received funding from a PFIS grant (FI18/00121). This work has been partially performed thanks to the 3T Equipment of Magnetic Resonance at IDIBAPS (project IBPS15-EE-3688 co funded by MCIU and by ERDF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Approval was obtained from Hospital Clínic Ethics Committee. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate/Consent for publication
Informed consent was obtained from all individual participants included in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Contador, J., Pérez-Millan, A., Guillen, N. et al. Baseline MRI atrophy predicts 2-year cognitive outcomes in early-onset Alzheimer’s disease. J Neurol 269, 2573–2583 (2022). https://doi.org/10.1007/s00415-021-10851-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10851-9