Abstract
Purpose
The aim of this study was to investigate the efficacy and safety of Bimatoprost Unit Dose Preservative Free (BUDPF) and Latanoprost Unit Dose Preservative Free (LUDPF).
Methods
A prospective, randomized, investigator-masked, cross-over comparison was used. Inclusion criteria were ocular hypertension (OHT) or open-angle glaucoma (OAG) with a maximum intraocular pressure (IOP) of 21 mmHg on a preserved prostaglandin monotherapy. After 6 weeks washout, patients were randomized to BUDPF or LUDPF for 3 months and then switched to the other treatment for 3 months. IOP curves were performed at baseline and after each treatment period. Statistical analysis was performed in a R programming environment. Linear mixed modeling was used to account for repeated measures on the same subject and clustering of observations from the same center. Safety outcomes included visual acuity, adverse events, slit-lamp biomicroscopy, ocular tolerability, and optic nerve assessment.
Results
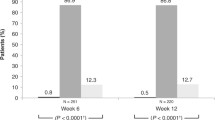
Analysis at 6 months (primary outcome) showed a 1.6 ± 0.5-mmHg difference in IOP values between LUDPF and BUDPF (p < 0.01). A mean intra-subject IOP difference of 0.9 ± 0.2 mmHg (LUDPF – BUDPF) was observed (p < 0.01).. Significant differences in IOP were observed for both drugs at 3 and at 6 months compared to baseline: −4,0 ± 0.5 mmHg for both BUDPF and LUDPF at 3 months (p < 0.01 for both drugs; p = 0.32 between the two drugs); −5.2 ± 0.5 and −3.4 ± 0.5 mmHg for BUDPF and LUDPF, respectively (both p < 0.01), at 6 months. Both drugs were tolerated well, the only statistically significant difference being lower hyperemia scores for LUDPF (albeit low for both drugs).
Conclusions
This study demonstrates a superior efficacy of BUDPF over LUDPF in lowering IOP. The results are consistent both in the parallel comparison between the two treatment groups at 6 months as well as in the intra-subject pressure comparison.



Similar content being viewed by others
References
Heijl A, Bengtsson B, Chauhan BC, Lieberman MF, Cunliffe I, Hyman L, Leske MC (2008) A comparison of visual field progression criteria of 3 major glaucoma trials in early manifest glaucoma trial patients. Ophthalmology 115:1557–1565. doi:10.1016/j.ophtha.2008.02.005
Gillespie BW, Musch DC, Guire KE, Mills RP, Lichter PR, Janz NK, Wren PA, CIGTS (Collaborative Initial Glaucoma Treatment Study) Study Group (2003) The collaborative initial glaucoma treatment study: Baseline visual field and test-retest variability. Invest Ophthalmol Vis Sci 44:2613–2620
Ernest PJ, Schouten JS, Beckers HJ, Hendrikse F, Prins MH, Webers CA (2013) An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology 120:512–519. doi:10.1016/j.ophtha.2012.09.005
Chauhan BC, Mikelberg FS, Balaszi AG, LeBlanc RP, Lesk MR, Trope GE Canadian Glaucoma Study Group; (2008) Canadian Glaucoma Study: 2. Risk factors for the progression of open-angle glaucoma. Arch Ophthalmol 126:1030–1036. doi:10.1001/archopht.126.8.1030
Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, Azuara-Blanco A, Bourne RR, Broadway DC, Cunliffe IA, Diamond JP, Fraser SG, Ho TA, Martin KR, McNaught AI, Negi A, Patel K, Russell RA, Shah A, Spry PG, Suzuki K, White ET, Wormald RP, Xing W, Zeyen TG (2015) Latanoprost for open-angle glaucoma (UKGTS): A randomised, multicentre, placebo-controlled trial. Lancet 385:1295–1304. doi:10.1016/S0140-6736(14)62111-5
van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH (2005) Intraocular pressure-lowering effects of all commonly used glaucoma drugs: A meta-analysis of randomized clinical trials. Ophthalmology 112:1177–1185
Aptel F, Cucherat M, Denis P (2008) Efficacy and tolerability of prostaglandin analogs: A meta-analysis of randomized controlled clinical trials. J Glaucoma 17:667–673. doi:10.1097/IJG.0b013e3181666557
Stalmans I, Sunaric Mégevand G, Cordeiro MF, Hommer A, Rossetti L, Goñi F, Heijl A, Bron A (2013) Preservative-free treatment in glaucoma: Who, when, and why. Eur J Ophthalmol 23:518–525. doi:10.5301/ejo.5000270
Day DG, Walters TR, Schwartz GF, Mundorf TK, Liu C, Schiffman RM, Bejanian M (2013) Bimatoprost 0.03% preservative-free ophthalmic solution versus bimatoprost 0.03% ophthalmic solution (Lumigan) for glaucoma or ocular hypertension: A 12-week, randomised, double-masked trial. Br J Ophthalmol 97:989–993. doi:10.1136/bjophthalmol-2012-303040
Rouland JF, Traverso CE, Stalmans I, Fekih LE, Delval L, Renault D, Baudouin C, T2345 Study Group (2013) Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br J Ophthalmol 97:196–200. doi:10.1136/bjophthalmol-2012-302121
Sanford M (2014) Preservative-free latanoprost eye drops in patients with primary open-angle glaucoma/ocular hypertension. Clin Drug Investig 34:521–528. doi:10.1007/s40261-014-0203-4
European Glaucoma Society (2014) Terminology and guidelines for glaucoma, 4th edn. Editrice Publicomm s.r.l, Savona
Parrish RK, Palmberg P, Sheu WP, XLT Study Group (2003) A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: A 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol 135:688–703
Noecker RS, Dirks MS, Choplin NT, Bernstein P, Batoosingh AL, Whitcup SM, Bimatoprost/Latanoprost Study Group (2003) A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol 135:55–63
Gandolfi S, Simmons ST, Sturm R, Chen K, VanDenburgh AM, Bimatoprost Study Group 3 (2001) Three-month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertension. Adv Ther 18:110–121
Cordeiro MF, Goldberg I, Schiffman R, Bernstein P, Bejanian M (2015) Efficacy of a preservative-free formulation of fixed-combination bimatoprost and timolol (Ganfort PF) in treatment-naïve patients vs previously treated patients. Clin Ophthalmol 9:1605–1611
Chauhan BC, Mikelberg FS, Artes PH, Balazsi AG, LeBlanc RP, Lesk MR, Nicolela MT, Trope GE, Canadian Glaucoma Study Group (2010) Canadian Glaucoma Study: 3 impact of risk factors and intraocular pressure reduction on the rates of visual field change. Arch Ophthalmol 128:1249–1255. doi:10.1001/archophthalmol.2010.196
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This investigator-initiated study was sponsored by the EVICR.net—European Vision Institute Clinical Research Network. Allergan provided financial support in the form of an unrestricted grant to EVICR.net.
Conflict of interest
Some of the authors have received financial support from companies that have interest in the subject of this study: consultancy fees (FC, GSM, TH, FO, LR, IS from Allergan and FC, IS, GSM from Théa pharma), honoraria for lectures (FC, GSM, TH, LR, IS from Allergan and FC, IS from Théa Pharma), and educational grants (LR, IS from Allergan, LR from Théa Pharma). The contribution of the IRCCS Fondazione Bietti in this paper was supported by the Italian Ministry of Health and by Fondazione Roma.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Stalmans, I., Oddone, F., Cordeiro, M.F. et al. Comparison of preservative-free latanoprost and preservative-free bimatoprost in a multicenter, randomized, investigator-masked cross-over clinical trial, the SPORT trial. Graefes Arch Clin Exp Ophthalmol 254, 1151–1158 (2016). https://doi.org/10.1007/s00417-016-3299-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3299-9