Abstract
Background
There are claims that ocular accommodation differs in children with attention deficit hyperactivity disorder (ADHD) compared to typically developing children. We examined whether the accommodation response in ADHD children is influenced by changing the stimulus to accommodation in an attempt modify the level of attentional engagement or by medication for the condition.
Methods
We measured the accommodative response and pupil diameter using a binocular, open-field autorefractor in non-medicated and medicated children with ADHD (n = 22, mean age = 10.1 ± 2.4 years; n = 19; mean age = 11.0 ± 3.8 years; respectively) and in an age-matched control group (n = 22; mean age = 10.6 ± 1.9 years) while participants were asked to maintain focus on (i) a high-contrast Maltese cross, (ii) a frame of a cartoon movie (picture) and (iii) a cartoon movie chosen by the participant. Each stimulus was viewed for 180 s from a distance of 25 cm, and the order of presentation was randomised.
Results
Greater lags of accommodation were present in the non-medicated ADHD in comparison to controls (p = 0.023, lags of 1.10 ± 0.56 D and 0.72 ± 0.57 D, respectively). No statistically significant difference in the mean accommodative lag was observed between medicated ADHD children (lag of 1.00 ± 0.44D) and controls (p = 0.104) or between medicated and non-medicated children with ADHD (p = 0.504). The visual stimulus did not influence the lag of accommodation (p = 0.491), and there were no significant group-by-stimulus interactions (p = 0.935). The variability of accommodation differed depending on the visual stimulus, with higher variability for the picture condition compared to the cartoon-movie (p < 0.001) and the Maltese cross (p = 0.006). In addition, the variability yielded statistically significant difference for the main effect of time-on-task (p = 0.027), exhibiting a higher variability over time. However, no group differences in accommodation variability were observed (p = 0.935).
Conclusions
Children with ADHD have a reduced accommodative response, which is not influenced by the stimulus to accommodation. There is no marked effect of medication for ADHD on accommodation accuracy.
Similar content being viewed by others
Introduction
Optometric examination requires cooperation on the part of the patient [1], and this can be particularly challenging in children. Maintaining a child’s attention is crucial to gathering accurate clinical measures which in turn will affect whether a correct diagnosis is made [2,3,4,5,6].
Attention deficit hyperactivity disorder (ADHD) is one of the most common psychological disorders diagnosed in childhood, with an estimated prevalence worldwide of 5.3% [7]. The condition is characterised by inattentive, impulsive and hyperactive symptoms [8]. There is an estimated prevalence of vision problems of ~ 16% associated with this disorder [9], with deficits in saccadic eye movements [10], ocular vergences [11], visual processing [12] and colour vision [13] being most often observed in individuals with this condition.
The accommodative response in children with ADHD has been measured using objective techniques, specifically an open-field autorefractor [14]. In this recent study, a greater lag of accommodation was observed in children with ADHD in comparison to age-matched controls. However, the reason for this finding is not clear. It is known that visual attention [15] and cognitive effort [16,17,18] influence the accuracy of the accommodative response. It is possible the result obtained by Redondo et al. stems from a primary accommodative deficit in this disorder [14], as has been previously observed in other neurological disorders such as Down syndrome [19] or autism [20]. On the other hand, it is also plausible that a less accurate accommodative response in children with ADHD stems from a lack of engagement, interest or motivation for the task. Several researchers have attempted to include an engaging stimulus (e.g. cartoon videos or games) in order to increase children’s attention and co-operation [21,22,23,24]. This is the approach taken here. We examine whether the accommodation response in children with ADHD is influenced by changing the stimulus to accommodation in an attempt to increase the level of attentional engagement.
A second aim is to compare the accommodative response in medicated versus non-medicated children with ADHD. Scientific evidence suggests that pharmacological treatment of ADHD with psychostimulants (e.g. methylphenidate and amphetamines) has a beneficial effect on the symptoms of hyperactivity, impulsivity and inattention [25], which seems to be mediated by increasing activation in dopamine and norepinephrine fronto-striatal circuitry [26, 27]. Only a few studies have assessed the effects of medication on the ocular function in ADHD. Methylphenidate may improve ocular motility [28, 29], visual field size and visual acuity [30] (but see [31]). Of note, Grönlund et al. [32] found a prevalence of 76% of ophthalmic abnormalities in children with ADHD, and this pattern did not significantly improve with pharmacological treatment. However, they observed that children with ADHD concentrated and cooperated better when they were medicated. To date, no studies have assessed the impact of ADHD treatment on the accommodative response in children with ADHD. Our aim here is therefore to examine the robustness of the finding that accommodation accuracy is reduced in children with ADHD [14] and the impact upon accommodation that medication for the condition may exert.
Methods
Participants
Based upon the results obtained by Redondo et al. [14], who obtained a partial eta squared of 0.14 for the between-subject comparison of the lag of accommodation, we conducted an a priori calculation for a repeated measures analysis of variance (ANOVA) with between- and within-factors. G*Power 3.1 was used software to calculate the minimal sample size required. This analysis projected that 18 subjects per group would be necessary when considering a power of 0.90 and an alpha of 0.05.
Twenty-three non-medicated (mean age = 10.1 ± 2.4 years) and 21 medicated children with ADHD (mean age = 11.0 ± 3.8 years), as well as 23 healthy controls (mean age = 10.6 ± 1.9 years), took part in this study. Children with ADHD were diagnosed by the Neuropsychology and Early Intervention Unit of San Cecilio University Hospital (Granada), using the Diagnostic and Statistical Manual of Mental Disorders 5th edition [8]. The ADHD-medicated children had been prescribed methylphenidate hydrochloride for at least 1 year. The initial dosage was 0.5 mg/kg/day; however, the dosage was later adjusted as a function of response and tolerance to treatment.
Children with an intelligence quotient lower than 85 were excluded [33]. Participants were screened according to the following inclusion criteria: (1) visual acuity of 0.10 logMAR or better in each eye, (2) no history of strabismus and/or amblyopia, (3) minimal un-corrected refractive error as determined by objective and subjective refraction (myopia of ≤ − 0.50, astigmatism and anisometropia of < 1.00 D and hyperopia ≤ 1.00 D) [34] and (4) no history of ocular disease. Sixteen of the participating children (six non-medicated children with ADHD, six medicated children with ADHD and four controls) wore their habitual refractive correction (mean refractive error = − 0.55 ± 1.56D). Five participants (1 non-medicated ADHD child, 2 medicated ADHD children and 2 controls) were excluded because they did not meet the inclusion criteria (2 strabismus, 1 amblyopia, 2 severe hyperopia). Thus, 22 non-medicated children with ADHD, 19 medicated children with ADHD and 22 controls were included in the analysis, who had a mean refraction or over refraction of 0.22 ± 0.36 D. All parents or guardians received detailed instructions and signed an informed consent. The protocol followed the tenets of the Declaration of Helsinki, and the study was approved by the University of Granada Institutional Review Board (546/CEIH/2018).
Manipulation of visual target engagement
Participants were asked to maintain focus on three different visual stimuli for 3 min each. The examiner gave written and verbal instructions to each child, indicating that they should keep the target in focus at all times. One consisted of a cartoon movie, which was chosen by participants from a range of ten available options (Heidi, SpongeBob, Dragon Ball, Adventure Time, Peppa Pig, Geronimo Stilton, Futurama, Robot Trains, Doraemon and Paw Patrol). A second stimulus was a picture taken from the cartoon movie they chose, and the still image had similar contrast and colour characteristics compared to the cartoon movie. Finally, a third stimulus consisted of a Maltese cross, which is a 2-cm, high-contrast (Michelson = 79%) five-point black-star target presented on a white background card. This stimulus contains a wide frequency spectrum with a suitable cue for accommodation studies [35]. All three stimuli were displayed on a smartphone (iPhone 4, Apple Inc., Cupertino, CA; screen resolution 640 × 960 pixels, 3.5-in.) in randomised order. Figure 1 shows an example of each of the three stimulus types used in this study.
Grand Seiko WAM-5500 autorefractor
Accommodative and pupil responses were measured with the open-field Grand Seiko Auto Refractometer WAM-5500 (Grand Seiko, Hiroshima, Japan), which is capable of acquiring reliable and valid measures [36]. Data recording was performed in the hi-speed mode (continuous recording mode) of the WAM-5500 at a temporal resolution of ~ 5 Hz. The visual stimulus was displayed on the centre of the smartphone screen at a distance of 25 cm (angular subtense of 10°) from the observer at his/her gaze height since it has shown to be children’s reading distance [37]. All measures were performed under binocular conditions, thus not eliminating convergent accommodation, and accommodation and pupil response data were taken from the dominant eye (determined by the hole-in-the card method) as recommended by Momeni-Moghaddam et al. [38].
For the analysis of the accommodative response, we identified and removed data points that were ± 3 standard deviations from the mean spherical refraction value, which could be due to blinking or recording errors [39]. The remaining data (average percentage 88%, range 82 to 93%) were used for further analyses. The accommodation deficit was determined for each participant and experimental condition by subtracting the accommodative response from the accommodative demand (4 D at 25 cm) after the refractive error had been considered [40]. For example, if the mean refractive error at 25 cm was 3.35 D, the mean lag of accommodation is 0.65 D, and if this subject is myopic of 0.10 D (after being optically corrected), then the mean accommodative lag would be 0.55 D instead of 0.65 D. When spectacle lenses were worn (six non-medicated children with ADHD, six medicated children with ADHD and four controls used an optical compensation [mean refractive error − 0.55 ± 1.56D]), we calculated the ocular accommodation demand referring to the corneal vertex for an assumed vertex distance of 12 mm [41]. The standard deviation of accommodation during each dynamic measurement was used to define the variability of accommodation.
Procedure
Firstly, clinical information about the children and family members was collected, and the presence of any ocular pathology was checked by slit lamp and direct ophthalmoscopy examination. Participants then underwent an optometric examination that included distance and near monocular and binocular visual acuity measurement, and non-cycloplegic objective refraction and over-refraction techniques, using an endpoint criterion of maximum-plus dioptric power consistent with best vision. When necessary, the children were compensated, and then, an objective monocular static refraction was performed in both eyes with the WAM-500 in order to determine the baseline refractive value (over-refraction in children wearing optical correction), which was used for the subsequent data analysis. All participants received the same instructions initially. The experimenters then checked that the child had understood and offered further instruction where this was not the case. Subsequently, accommodation and pupil data were continuously recorded while viewing each stimulus in turn for 3 min. During the dynamic accommodation recording, the examiner ensured that the instrument remained carefully aligned with the visual target in order to acquire on-axis measurements. A 3-min break was given between conditions in order to avoid changes in tonic accommodation due to sustained accommodative or convergence effort [42]. To assess the effect of time-on-task, all the dependent variables were divided into three consecutive 60-s blocks. The luminance of the smartphone screen was 38 cd/m2 (PR-745 SpectraScan Spectroradiometer, Photo Research Inc., Chatsworth, CA). All measurements were obtained under the same illumination conditions (~ 150 lx as measured in the corneal plane; Illuminance meter T-10, Konica Minolta, Inc., Japan).
Experimental design and statistical analyses
The study followed a 3 × 3 × 3 mixed factorial design. We considered the Group (non-medicated ADHD, medicated ADHD and control) as the between-participant factor and the visual stimulus (Maltese cross, picture and cartoon movie) and the time-on-task (block1, block 2 and block 3) as the within-participant factors. The dependent variables were the accommodation deficit (lag or lead of accommodation), variability of accommodation, pupil diameter and variability of pupil diameter.
Prior to statistical analysis, the normal distribution of the data (Shapiro-Wilk test) and the homogeneity of variances (Levene’s test) were confirmed (p > 0.05). To explore the possible differences in age between the experimental groups, we performed a ANOVA with the Group (non-medicated ADHD, medicated ADHD and control) as the only between-participant factor. Then, separate mixed ANOVAs were carried out for each dependent variable (accommodation deficit, variability of accommodation, pupil diameter and variability of pupil diameter). We reported partial eta squared (ƞp2) and Cohen’s d (d) as effect size indices for Fs and t tests, respectively. Statistical significance was set at an alpha level of 0.05, and post-hoc tests were corrected with Holm-Bonferroni procedure.
Results
Descriptive values (mean and standard deviation) for the accommodation and pupil response in the three groups for each accommodative stimulus are shown in Table 1.
A unifactorial ANOVA to test for possible age differences between groups revealed no statistically significant age differences between the three groups (F57 = 0.79, p = 0.492, η2 = 0.025). To assess the possible differences in the amount of remaining data (excluding blinking and recording errors), a mixed ANOVA was performed. This analysis revealed that there were no statistically significant differences for any main or interactive effect (all p values > 0.05).
The analysis of the accommodation deficit exhibited significant effects for group (F2, 60 = 4.073, p = 0.022, η2 = 0.120) and for the time-on-task × group interaction (F4, 120 = 0.717, p = 0.006, η2 = 0.112). Separate unifactorial ANOVAs for each visual stimulus and group showed no significant effects for time-on-task (p values > 0.05 in all cases). No statistically significant differences were observed for the visual stimulus (F4, 120 = 0.715, p = 0.491, η2 = 0.012) or for any other main or interactive effects (p values > 0.05 in all cases). Post-hoc analyses revealed a greater lag of accommodation for the non-medicated children with ADHD in comparison to controls (corrected p value = 0.023, d = 0.347); however, no statistically significant difference in accommodation deficit was observed between medicated ADHD children and controls (corrected p value = 0.104, d = 0.250) or between medicated and non-medicated children with ADHD (corrected p value = 0.504, d = 0.085) (Fig. 2).
Accommodation deficits for the non-medicated ADHD, medicated ADHD and control groups while viewing the three stimulus types. Positive values indicate lags of accommodation, whereas negative values show leads of accommodation. The box plots represent 75th and 25th centiles, and individual data are displayed as jittered dots. The horizontal line into the box indicates the median value. The whiskers show the range of values within the 1.5× interquartile range
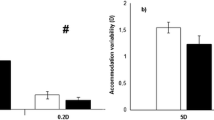
The variability of accommodation yielded statistically significant differences depending on the visual stimulus (F2, 120 = 8.433, p < 0.001, η2 = 0.123), and time-on-task (F2, 120 = 3.723, p = 0.027, η2 = 0.058), but no differences were obtained for the main factor of group (F2, 60 = 0.067, p = 0.935, η2 = 0.002) or for any interaction (p value > 0.05). Post-hoc analyses exhibited a higher variability of accommodation for the picture condition in comparison to the Maltese cross (corrected p value = 0.006, d = 0.389) and for the picture compared to the cartoon movie conditions (corrected p value < 0.001, d = 0.499). However, there was no difference when the Maltese cross was compared with the cartoon movie condition (corrected p value = 0.355, d = 0.117) (Fig. 3). Although time-on-task was a significant factor, post-hoc analyses for the comparison between the three 60-s blocks did not show statistically significant differences; however, there was a trend toward higher variability over time (block 3 vs. block 1: corrected p value = 0.770; block 3 vs. block 1: corrected p value = 0.056; and block 3 vs. block 2: corrected p value = 0.056).
Variability of accommodation for the non-medicated ADHD, medicated ADHD and control groups while viewing the three stimulus types. The box plots represent 75th and 25th centiles, and individual data are displayed as jittered dots. The horizontal line into the box indicates the median value. The whiskers show the range of values within the 1.5× interquartile range
Pupil diameter and variability of pupil diameter did not exhibit statistically significant differences for the main factors of visual stimulus, time-on-task or group (p value > 0.05 in all cases).
Discussion
The present study was aimed at determining the origin of the impaired accommodative response in children with ADHD [14], as well as the possible influence of stimulant medication for ADHD on accommodation. We hypothesised that if there is a primary deficit in accommodation, the differences in accommodative response will be independent of the stimulus used to determine the accommodative response, whereas if the accommodative deficit is a consequence of the attention disorder, the magnitude of the accommodative deficit may be reduced when the child is more engaged [21, 43]. In common with previous research conducted by this group [14], our results reveal a greater mean lag of accommodation in non-medicated children with ADHD compared to the control group. However, no difference in lag was found between medicated children with ADHD and the controls. Regardless of group, the lag of accommodation was not influenced by the accommodative stimulus. By contrast, the variability of accommodation was dependent on the stimulus, yielding less stable accommodation for the picture condition, but again there were no between-group differences. Since the magnitude of the lag was not affected by the stimulus to accommodation, we conclude that either non-medicated ADHD children exhibit a primary accommodative deficit or that our attempts to increase the attentional engagement by changing the stimulus to accommodation were unsuccessful. We address which of these is the more likely in the text below.
We found that children with ADHD had a mean lag of around 0.5 D greater than the control group when fixating the Maltese cross at 25 cm (see Table 1). Our results agree with a previous study that found a higher lag of accommodation in a population of non-medicated children with ADHD compared to a healthy, age-matched control group (approximately 0.5 D greater in the ADHD group at 20 cm [14]. Inaccurate accommodation may lead to asthenopia and impair behavioural performance [44], which might explain the greater near symptomatology (e.g. convergence insufficiency) reported by children with ADHD [45, 46]. Normative data indicate that an accommodative lag, as measured by MEM retinoscopy at 40 cm, of approximately 0.50 ± 0.40 D may be expected in 10-year-old children [47]. Here, we found a mean accommodative lag, using an open-field autorefractor, of 1.19 ± 0.57 D for the Maltese cross at 25 cm in children with ADHD (see Table 1), and thus, our results may be considered clinically as well as statistically significant.
The accommodative response is not stable when focusing on a stationary target and typically fluctuates by ~ 0.5 D around the mean response [48]. We did not find any group difference in the variability of accommodation, which agrees with Redondo et al., who also found differences between the ADHD and a control group in the lag of accommodation, and not in the variability of their accommodation. Roberts et al. observed that the variability of accommodation was lower while performing an active sustained task of 10 min compared to a passive and non-engaging task in children. Similarly, our results show that the variability of the accommodative response is dependent on the visual stimulus; all children exhibited a higher variability for the picture condition in comparison to the cartoon movie, which we attribute to the fact watching moving images is a more engaging task. In addition, there was a trend toward higher variability of accommodation over time, with these effects being evident in the last minute of the 3-min viewing task. Previous studies have reported stable behaviour of the accommodative response over time in an ADHD and control population [14, 39], which may seem contradictory to the present findings. However, both of the aforementioned studies recorded accommodation for a 90-s period compared to the 180 s recording period employed here. Our results suggest that the stability of accommodation can be modulated by increasing the attractiveness of the stimulus [18]. Given that this occurred regardless of group, it is tempting to suggest that we have increased the attentional engagement in all children, including ADHD children, and in turn suggests that the greater lag of accommodation in the non-medicated ADHD children may be a primary deficit of accommodation rather a failure to engage the children with the movie presentation. However, future studies that objectively analyse the attentional state (e.g. using electroencephalography) are needed to test whether this assertion is correct.
It is well known that changes in pupil diameter and accommodation are strongly correlated but not necessarily causally related [49]. The pupillary response provides information about autonomic nervous system and has also been shown to be sensitive to changes in the attentional state [50, 51]. In agreement with Kara et al. [52], we did not find significant differences between the ADHD and control groups for the magnitude or variability of pupil diameter, suggesting that these variables may not be sensitive enough to be used as physiological markers for diagnosis of ADHD. Here, the analysis of the pupil dynamics was carried out in order to explore whether the changes in the accommodative response may be explained by variations in the pupil behaviour. However, the lack of group differences for the pupillary response indicates that changes in ocular accommodation were not linked to pupil diameter variations in this study. Regarding effects of stimulant medication, the sympathomimetic action of methylphenidate may lead to pupil dilation. A recent study observed larger pupil diameters in children with ADHD when using stimulant medication, although these effects might be partially explained by the session order (i.e. the order of both sessions was not counterbalanced) [53].
The sustained use of methylphenidate has been shown to induce changes in brain neurochemistry, modifying the cognitive and neural functioning [54]. Methylphenidate affects the prefrontal cortex and striatum and acts by blocking both dopamine and norepinephrine transporters, which leads to increased extracellular dopamine and norepinephrine (Arnsten, 2006). However, the effect of methylphenidate on visual function is not well established with mixed results in the scientific literature [28,29,30,31,32, 55]). In this regard, the use of methylphenidate has been linked by the European Medicines Agency with several changes in visual function, including mydriasis and accommodation difficulties, although its incidence is rare [55]. In our study, the medicated ADHD group exhibited mean accommodative lags that were not different to those in the non-medicated ADHD group (Table 1) and the same is true of the mean accommodative lags in the medicated ADHD group and typically developing children. These results suggest, therefore, that the use of stimulant medication has at best a minor effect on accommodation accuracy in children with ADHD, which could mean that the brain areas (e.g. superior colliculus) controlling the near response [56] are unaffected by the stimulant medication. Other studies observed improvements in blink and microsaccade rates and visual fields when children with ADHD were treated with stimulants [28,29,30, 57,58,59]. However, these visual functions are controlled by different physiological mechanisms than those responsible for driving the dynamics of the accommodative response [60]. Based on the results of our study, the use of stimulants (i.e. methylphenidate) in treatment of ADHD medication will probably not eliminate the accommodative deficits found in children with this neuropsychological disorder.
Our study has a number of limitations. First, the screen luminance and colour content were not matched between the Maltese cross, picture and cartoon movie conditions, and this may lead to some differences in the accommodative response behaviour [61] since the accommodation response is sensitive to luminance and chromatic components of the stimulus [62]. Although, it should be noted that there were no differences in pupil diameter between conditions. Second, the angular subtense for the three visual stimuli was the same (10°). However, fixation stability was not controlled in the current investigation, and hence, we cannot discard the possibility that differences in fixation stability may partially explain the differences we observed in the accommodative response (lag and variability of accommodation). Third, although we found a greater lag of accommodative in the non-medicated ADHD group, we cannot establish the physiological cause for this finding. It is our hope that future studies will determine the brain mechanisms responsible for the impaired accommodative function in children with ADHD. Fourth, the pharmacological treatment does not seem to substantially improve the accommodative function in the ADHD population, and thus, the effectiveness of alternative treatment strategies such as glasses or visual therapy should be investigated. In addition, there is evidence that some comorbidities or ADHD symptoms cannot be exclusively addressed by the stimulant medication, supporting that the development of new drugs is necessary to manage the symptoms and signs associated with ADHD [27]. Therefore, the impact of different pharmacological interventions on the accommodative function should be explored in future investigations.
Conclusions
This study provides further evidence for an impaired accommodative response in children with ADHD. We found that non-medicated children with ADHD present a higher mean accommodative lag than age-matched controls, with this effect being independent of the stimulus to accommodation, even when these are designed to increase engagement. Methylphenidate treatment seems not to have a marked effect on the mean accommodative lag. The variability of accommodation was affected by the visual stimulus, showing a more stable accommodative response while viewing the more engaging task (cartoon movie). However, non-medicated and medicated children with ADHD and healthy controls exhibited similar stability of accommodation. Taken together, the present findings reveal novel insights into the inherent accommodative deficit of children with ADHD and they suggest that the lack of accuracy in accommodative response in non-medicated ADHD children may result from a primary deficit associated with this condition.
References
Scheiman M, Wick B (2008) Clinical management of binocular vision: heterophoric, accommodative, and eye movement disorders, 3rd edn. Lippincott Williams & Wilkins, Philadelphia
Piast M, Kustrzeba-Wójcicka I, Matusiewicz M, Banaś T (2003) A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin Neurophysiol 114:184–198. https://doi.org/10.1016/s1388-2457(02)00363-2
Coulter RA, Shallo-Hoffmann J (2000) The presumed influence of attention on accuracy in the developmental eye movement (DEM) test. Optom Vis Sci 77:428–432
Börger N, van der Meere J (2000) Visual behaviour of ADHD children during an attention test: an almost forgotten variable. J Child Psychol Psychiatry Allied Discip 41:525–532
Yeshurun Y, Carrasco M (1998) Attention improves or impairs visual performance by enhancing spatial resolution. Nature 396(6706):72–75
Ishikawa M, Yoshimura M, Sato H, Itakura S (2019) Effects of attentional behaviours on infant visual preferences and object choice. Cogn Process. https://doi.org/10.1007/s10339-019-00918-x
Polanczyk G, De Lima MS, Horta BL et al (2007) The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164:942–948. https://doi.org/10.1176/appi.ajp.164.6.942
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Washington, DC
Akmatov MK, Ermakova T, Bätzing J (2019) Psychiatric and nonpsychiatric comorbidities among children with ADHD: an exploratory analysis of nationwide claims data in Germany. J Atten Disord 108705471986577. https://doi.org/10.1177/1087054719865779
Rommelse N, Stigchel S, Sergeant J (2008) A review on eye movement studies in childhood and adolescent psychiatry. Brain Cogn 68:391–414. https://doi.org/10.1016/j.bandc.2008.08.025
Puig M, Zapata L, Puigcerver L, Iglesias NE (2015) Attention-related eye vergence measured in children with attention deficit hyperactivity disorder. PLoS One:1–16. https://doi.org/10.1371/journal.pone.0145281
Lenz D, Krauel K, Flechtner H et al (2010) Neuropsychologia altered evoked gamma-band responses reveal impaired early visual processing in ADHD children. Neuropsychologia 48:1985–1993. https://doi.org/10.1016/j.neuropsychologia.2010.03.019
Kim S, Banaschewski T, Tannock R (2014) Color vision in attention-deficit / hyperactivity disorder: a pilot visual evoked potential study. J Optom 8:116–130. https://doi.org/10.1016/j.optom.2014.10.002
Redondo B, Vera J, Molina R, García JA, Ouadi M, Muñoz-Hoyos A, Jiménez R (2018) Attention-deficit/hyperactivity disorder children exhibit an impaired accommodative response. Graefes Arch Clin Exp Ophthalmol 256(5):1023–1030
Lawson ML, Crewther SG, Junghans BM, Crewther DP, Kiely PM (2005) Changes in ocular accommodation when shifting between global and local attention. Clin Exp Optom 88:28–32
Davies LN, Wolffsohn JS, Gilmartin B (2005) Cognition, ocular accommodation, and cardiovascular function in emmetropes and late-onset myopes. Investig Ophthalmol Vis Sci 46:1791–1796. https://doi.org/10.1167/iovs.04-0986
Rosenfield M, Ciuffreda KJ (1990) Proximal and cognitively-induced accommodation. Ophthalmic Physiol Opt 10:252–256
Roberts TL, Manny RE, Benoit JS, Anderson HA (2018) Impact of cognitive demand during sustained near tasks in children and adults. Optom Vis Sci 95:223–233. https://doi.org/10.1097/OPX.0000000000001186
Woodhouse JM, Cregg M, Gunter HL et al (2000) The effect of age, size of target, and cognitive factors on accommodative responses of children with down syndrome. Investig Ophthalmol Vis Sci 41:2479–2485
Anketell PM, Saunders KJ, Gallagher SM, Bailey C (2018) Accommodative function in individuals with autism spectrum disorder. Optom Vis Sci 95:193–201. https://doi.org/10.1097/OPX.0000000000001190
Gaggi O, Ciman M (2016) The use of games to help children eyes testing. Multimed Tools Appl 75:3453–3478. https://doi.org/10.1007/s11042-014-2444-x
Aslam TM, Rahman W, Henson DKP (2011) A novel paediatric game-based visual-fields assessor. Br J Ophthalmol 95:921–924
Wang Y, Ali Z, Subramani S et al (2017) Normal threshold size of stimuli in children using a game-based visual field test. Ophthalmol Therapy 6:115–122. https://doi.org/10.1007/s40123-016-0071-5
Anderson HA, Glasser A, Manny RE, Stuebing KK (2010) Age-related changes in accommodative dynamics from preschool to adulthood. Investig Ophthalmol Vis Sci 51:614–622. https://doi.org/10.1167/iovs.09-3653
Greenhill L (2001) Clinical effects of stimulant medication in ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX (eds) Stimulant drugs and ADHD: basic and clinical neuroscience. Oxford University Press, NewYork
Arnsten AF (2006) Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology 31:2376
Molina-Carballo A, Checa-Ros A, Muñoz-Hoyos A (2016) Treatments and compositions for attention deficit hyperactivity disorder: a patent review. Expert Opin Ther Pat 26:799–814. https://doi.org/10.1080/13543776.2016.1182989
Fried M, Tsitsiashvili E, Bonneh YS et al (2014) ADHD subjects fail to suppress eye blinks and microsaccades while anticipating visual stimuli but recover with medication. Vis Res 101:62–72. https://doi.org/10.1016/j.visres.2014.05.004
Klein CH, Raschke A, Brandenbusch A (2003) Development of pro- and antisaccades in children with attention-deficit hyperactivity disorder (ADHD) and healthy controls. Psychophysiology 40:17–28
Martin L, Aring E, Landgren M et al (2008) Visual fields in children with attention-deficit/hyperactivity disorder before and after treatment with stimulants. Acta Ophthalmol 86:259–264. https://doi.org/10.1111/j.1755-3768.2008.01189.x
Whitman BY, Lindsay RL, Cruz OAP (1997) The effect of ritalin (methylphenidate) on visual acuity of children treated for attention- deficit/hyperactivity disorder. Pediatr Res 41:100
Grönlund MA, Aring E, Landgren M, Hellström A (2007) Visual function and ocular features in children and adolescents with attention deficit hyperactivity disorder, with and without treatment with stimulants. Eye 21:494–502. https://doi.org/10.1038/sj.eye.6702240
Wechsler D (1974) Manual for the Wechsler intelligence scale for children, revised. Psychological Corporation, New York
Kulp MT, Ciner E, Maguire M et al (2017) Attention and visual motor integration in young children with uncorrected hyperopia. Optom Vis Sci 94:1. https://doi.org/10.1097/OPX.0000000000001123
Charman WN, Tucker J (1977) Dependence of accommodation response on the spatial frequency spectrum of the observed object. Vis Res 17:129–139
Sheppard AL, Davies LN (2010) Clinical evaluation of the Grand Seiko Auto Ref/Keratometer WAM-5500. Ophthalmic Physiol Opt 30:143–151. https://doi.org/10.1111/j.1475-1313.2009.00701.x
Wang Y, Bao J, Ou L et al (2013) Reading behavior of emmetropic schoolchildren in China. Vis Res 86:43–51. https://doi.org/10.1016/j.visres.2013.03.007
Momeni-Moghaddam H, McAlinden C, Azimi A et al (2014) Comparing accommodative function between the dominant and non-dominant eye. Graefes Arch Clin Exp Ophthalmol 252:509–514. https://doi.org/10.1007/s00417-013-2480-7
Tosha C, Borsting E, Ridder WH, Chase C (2009) Accommodation response and visual discomfort. Ophthalmic Physiol Opt 29:625–633. https://doi.org/10.1111/j.1475-1313.2009.00687.x
Poltavski D, Biberdorf D, Petros T (2012) Accommodative response and cortical activity during sustained attention. Vis Res 63:1–8. https://doi.org/10.1016/j.visres.2012.04.017
Atchison DA, Varnas SR (2017) Accommodation stimulus and response determinations with autorefractors. Ophthalmic Physiol Opt 37:96–104. https://doi.org/10.1111/opo.12340
Hasebe S, Graf EW, Schor CM (2001) Fatigue reduces tonic accommodation. Ophthalmic Physiol Opt 21:151–160. https://doi.org/10.1046/j.1475-1313.2001.00558.x
Horwood AM, Riddell PM (2010) Differences between naïve and expert observers’ vergence and accommodative responses to a range of targets. Ophthalmic Physiol Opt 30:152–159. https://doi.org/10.1111/j.1475-1313.2009.00706.x
Sterner B, Gellerstedt M, Sjöström A (2006) Accommodation and the relationship to subjective symptoms with near work for young school children. Ophthalmic Physiol Opt 26:148–155. https://doi.org/10.1111/j.1475-1313.2006.00364.x
Borsting E, Rouse M, Chu R (2005) Measuring ADHD behaviors in children with symptomatic accommodative dysfunction or convergence insufficiency: a preliminary study. Optometry 76:588–592
Granet DB, Gomi CF, Ventura RE, Miller-Scholte A (2005) The relationship between convergence insufficiency and ADHD. Strabismus 13:163–168. https://doi.org/10.1080/09273970500455436
Jiménez R, Gonzalez MD, Pérez MA, García JA (2003) Evolution of accommodative function and development of ocular movements in children. Ophthalmic Physiol Opt 23:97–107
Charman WN, Heron G (2015) Microfluctuations in accommodation: an update on their characteristics and possible role. Ophthalmic Physiol Opt 35:476–499. https://doi.org/10.1111/opo.12234
Miller NR (1985) Walsh and Hoyt’s clinical neuro-ophthalmology. Williams & Wilkins, Baltimore
Unsworth N, Robison MK (2016) Pupillary correlates of lapses of sustained attention. Cogn Affect Behav Neurosci 16:601–615. https://doi.org/10.3758/s13415-016-0417-4
Donaghue KC, Pena MM, Fung ATW, Bonney M, Howard NJ, Silink M, Schwingshandl J (1995) The prospective assessment of autonomic nerve function by pupillometry in adolescents with type 1 diabetes mellitus. Diabet Med 12:868–873
Kara K, Karaman D, Erdem U et al (2013) Investigation of autonomic nervous system functions by pupillometry in children with attention deficit hyperactivity disorder. Klin Psikofarmakol Bülteni-Bulletin Clin Psychopharmacol 23:49–56. https://doi.org/10.5455/bcp.20121130085850
Wainstein G, Rojas-Líbano D, Crossley NA et al (2017) Pupil size tracks attentional performance in attention-deficit/hyperactivity disorder. Sci Rep 7:1–9. https://doi.org/10.1038/s41598-017-08246-w
Gray JD, Punsoni M, Tabori NE et al (2007) Methylphenidate administration to juvenile rats alters brain areas involved in cognition, motivated behaviors, appetite, and stress. J Neurosci 27:7196–7207. https://doi.org/10.1523/JNEUROSCI.0109-07.2007
Larrañaga-Fragoso P, Noval S, Rivero JC, Boto-De-Los-Bueis A (2015) The effects of methylphenidate on refraction and anterior segment parameters in children with attention deficit hyperactivity disorder. J AAPOS 19:322–326. https://doi.org/10.1016/j.jaapos.2015.04.005 https://www.ema.europa.eu/en/documents/referral/methylphenidate-article-31-referral-annex-i-ii-iii-iv_en.pdf
Suzuki Y (2007) The near response: the contributions of Kenji Ohtsuka, MD. J Neuroophthalmol 27:138–142
Lu C, Kuang T, Chou JC (2006) Methylphenidate (ritalin)-associated cataract and glaucoma. J Chin Med Assoc 69:589–590. https://doi.org/10.1016/S1726-4901(09)70335-1
Tobaiqy M, Helms PJ, Williams J et al (2011) Parental reporting of adverse drug reactions associated with attention-deficit hyperactivity disorder (ADHD) medications in children attending specialist paediatric clinics in the UK. Drug Saf 34:211–219. https://doi.org/10.2165/11586050-000000000-00000
Soyer J, Jean-Louis J, Ospina LH et al (2019) Visual disorders with psychostimulants: a paediatric case report. Paediatr Child Health 24:153–155. https://doi.org/10.1093/pch/pxz012
Skalicky S (2016) Ocular and visual physiology: clinical application. Springer, Singapore
Ward PACW (1985) Effect of pupil size on steady-state accommodation. Vis Res 25:1317–1326
Rucker FJ, Kruger PB (2004) The role of short-wavelength sensitive cones and chromatic aberration in the response to stationary and step accommodation stimuli. Vis Res 44:197–208. https://doi.org/10.1016/j.visres.2003.09.011
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Granada Institutional Review Board (IRB approval: 546/CEIH/2018) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
As the participants of this study were minors, informed consent was obtained from their parents or guardians.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Redondo, B., Molina, R., Vera, J. et al. Accommodative response in children with attention deficit hyperactivity disorder (ADHD): the influence of accommodation stimulus and medication. Graefes Arch Clin Exp Ophthalmol 258, 1299–1307 (2020). https://doi.org/10.1007/s00417-020-04645-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04645-4