Abstract
Purpose
This study aimed to evaluate the effectiveness of bench step (BS) exercise for ameliorating arterial stiffening caused by acute mental stress (MS).
Methods
Fifteen young healthy men participated in two randomized trials: rest (RE) and exercise (EX) trials. Following a 5-min MS task (first task), the RE trial participants rested on a chair for 10 min (from 10 to 20 min after task cessation); the EX trial participants performed BS exercise for the same duration. At 40 min after the first task, the participants performed the same task (second task) again. Heart–brachial pulse wave velocity (PWV) (hbPWV), brachial–ankle PWV (baPWV), heart–ankle PWV (haPWV), and the cardio-ankle vascular index (CAVI) were measured simultaneously at 5, 30, and 50 min after the first task.
Results
Both trials caused significant elevations in hbPWV, haPWV, and CAVI at 5 min after the first task; these changes persisted until 30 min after the task in the RE trial, while they were abolished in the EX trial. baPWV significantly increased at 30 min after the task in the RE trial, but not in the EX trial. After the second task (from 30 to 50 min after the first task), none of the parameters significantly increased in the RE trial, although the values remained above baseline levels. In the EX trial, hbPWV, haPWV, and CAVI showed significant elevations.
Conclusion
Our findings suggest that a 10-min BS exercise after acute MS can counteract stress-induced arterial stiffening, but has only a limited effect against subsequent acute MS.
Similar content being viewed by others
Introduction
Mental stress (MS), which is common in daily life, is a known cause of deterioration of vascular health (Kivimäki and Steptoe 2018). Even a brief acute episode of MS results in a transient increase in arterial stiffness (Kume et al. 2020, 2021; Vlachopoulos et al. 2006); this would be attributed to various physiological factors, such as changes in endothelial function, sympathetic nerve activity, hormonal status, inflammation, and oxidative stress (Kume et al. 2020, 2021; Poitras and Pyke 2013; Vlachopoulos et al. 2006, 2009). Since it has been suggested that both acute response and chronic adaptation of the vasculature to MS are probably relevant (Chida and Steptoe 2010; Lima et al. 2019), chronic repetition of episodic increases in arterial stiffness due to acute MS in daily life may contribute to persistent arterial stiffness. In this context, it is important to devise an effective strategy for ameliorating arterial stiffening associated with acute MS to maintain vascular health.
We have recently shown that acute MS-caused arterial stiffening can be reversed by incorporating aerobic exercise on a cycle ergometer for only 10 min thereafter (Kume et al. 2021). Such an exercise strategy is advantageous in terms of vascular health maintenance. However, the applicability of exercise on a cycle ergometer in real life is relatively limited; thus, more easily accessible practical exercises are warranted as a countermeasure for acute MS-induced arterial stiffening. Bench step (BS) exercise can be conveniently performed at a variety of locations, such as the office and home, without the need for special equipment; the exercise can also be easily performed using stairs. Because regular BS exercise training ameliorates the basal arterial stiffness levels (Ohta et al. 2012), it is possible that acute BS exercise can be used as a countermeasure for the detrimental vascular effects of acute MS. This aspect, however, has not been clarified.
Traditionally, aerobic exercise has been known to positively modulate cardiovascular response to subsequent acute MS, including an attenuated increase in blood pressure (BP) during stress (Hamer et al. 2006). Sales et al. (2014) have recently reported that a 50-min exercise session prevented an impairment of endothelial function resulting from subsequent acute MS. Because arterial stiffness is largely regulated by endothelial function (McEniery et al. 2006), exercise may have a similar beneficial effect on arterial stiffness response to subsequent acute MS.
Based on this background, the present study aimed to evaluate the effectiveness of BS exercise for ameliorating acute MS-induced arterial stiffening. To achieve this objective, we first examined the effect of a single bout of 10-min BS exercise on arterial stiffness (experiment 1). Second, we investigated whether the 10-min BS exercise after acute MS would counteract arterial stiffening caused by stress and further examined the effect of exercise on the arterial stiffness response to subsequent acute MS (experiment 2).
Methods
Participants
We calculated the sample size required for this study using G*Power 3.1 power calculations. Based on our previous studies (Kume et al. 2020, 2021), the effect size was set as 0.25 using a within-between interaction of two-way repeated-measures analysis of variance (ANOVA). The α- and β-levels were set to 0.05 and 0.2 (80% power), respectively. The minimum number of participants required was 12.
Fifteen young healthy men (means ± SE; age: 21.6 ± 0.2 years; height: 170.8 ± 1.1 cm; body weight: 60.9 ± 1.3 kg) participated in experiment 1. In experiment 2, 15 healthy young men (age: 21.9 ± 0.3 years; height: 171.4 ± 1.1 cm; body weight: 62.4 ± 1.0 kg), including 12 individuals who participated in experiment 1, were studied. None of the participants were smokers, and none were taking medications. All participants provided written informed consent, and the study purpose, experimental procedure, and associated risks were fully explained to them. The study was approved by the Human Ethics Committee at the Osaka Institute of Technology (#2021-10) and was conducted in accordance with the guidelines of the Declaration of Helsinki.
Experimental procedures
Experiments 1 and 2 were conducted at least 3 months apart in each participant subjected to both experiments. In both experiments, the participants visited three times throughout the experimental period. During the first visit, the participants were allowed to familiarize themselves with the experimental apparatus. Subsequently, a 5-min resting heart rate (HR) measurement was taken while the participant was seated on a comfortable chair; the participants then engaged in a submaximal incremental exercise test using a step bench (Stepwell 2; Konami, Tokyo, Japan). The height of the step bench was 20 cm and the step rhythm was controlled by an electronic metronome. During the exercise test, HR was recorded continuously. The resting HR value and step rhythm-HR data were used to determine the step rate for the subsequent experimental visit.
During the second and third visits (i.e., experimental visits), the participants were subjected to exercise (EX) and rest (RE) trials in a random order, which was separated by approximately 1 week. All experiments were conducted in a quiet air-conditioned room (24–26 ºC). To avoid any potential diurnal effects, the experiments were conducted at the same time of the day for each participant. The participants were asked to refrain from performing strenuous exercise, consuming alcohol (≥ 24 h) and caffeine (≥ 12 h), and eating (≥ 3 h) before the trials.
Experiment 1
A schematic representation of the experimental protocol for experiment 1 is presented in Fig. 1. Baseline measures of arterial stiffness and hemodynamic variables were obtained from the participants in the supine position after a 15-min rest period. Subsequently, the participants in the RE trial rested on a chair for 10 min, whereas those in the EX trial performed BS exercise at 35–40% of HR reserve (HRR) for the same duration. This is considered as light-intensity aerobic exercise (Garber et al. 2011) and was chosen because of the potentially high applicability in daily life. The targeted HR range was calculated using the Karvonen formula: targeted HR range = [220—age—resting HR] × 0.35 or 0.40 (exercise intensity 35–40%) + resting HR. The exercise was started at a predetermined step rhythm while monitoring HR using a three-lead electrocardiogram (ECG) (413; Intercross, Tokyo, Japan); the step rhythm was adjusted manually, if necessary, to maintain the targeted HR value (Kume et al. 2021). After the seated rest or exercise period, the participants were allowed to recover in the supine position for 30 min. This recovery period was set because a decrease in arterial stiffness was reported to last for 30 min after short-term cycling exercise (Zhou et al. 2015).
Experiment 2
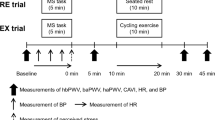
A schematic representation of the experimental protocol for experiment 2 is shown in Fig. 2. The baseline measurements were taken in the same manner as in experiment 1, and the participants then performed a 5-min MS task (first task) in both trials. Subsequently, from 10 to 20 min after the first task, the participants either rested while being seated on a chair (RE trial) or performed BS exercise (EX trial) using the same procedure as in experiment 1. From 40 min after the cessation of the first task, the participants were again exposed to the MS task (second task). Except for the seated rest or exercise periods, the participants remained in supine position throughout the experimental session.
Based on our previous studies (Kume et al. 2020, 2021), we used mental arithmetic as the MS task. The participants were asked to serially subtract 13 from a three-digit number as quickly and accurately as possible for a period of 5 min. During the task, the participants were asked to perform faster and were corrected immediately if wrong answers were provided to intentionally cause frustration. A metronome was played loudly for additional distraction. When the number was < 13 (the answer was not allowed to go below 0), the participants restarted the task using the original three-digit number. Each participant completed four MS tasks with a different starting number in a random order.
Measurements
Experiment 1
At baseline and every 10 min after the seated rest or exercise, pulse wave velocity (PWV), an established marker of arterial stiffness, HR, systolic and diastolic BP (SBP and DBP), and mean arterial pressure (MAP) were measured using a vascular testing system (VaSera VS-1500AN; Fukuda Denshi, Tokyo, Japan). For the measurements, BP cuffs were wrapped around both the upper arms and ankles. A phonocardiograph and ECG electrodes were placed on the pectoral region and on both wrists, respectively. ECG, heart sounds, and arterial pressure waveforms at the brachial and posterior-tibial arteries were simultaneously recorded. According to our previous study, we measured heart–brachial PWV (hbPWV), brachial–ankle PWV (baPWV), and heart–ankle PWV (haPWV) (Kume et al. 2020, 2021). Specifically, hbPWV, baPWV, and haPWV were calculated from each arterial path length along with the time intervals between the second heart sound and the dicrotic notch on the brachial arterial pressure waveform, between the foot of the brachial arterial pressure waveform and the foot of the posterior-tibial arterial waveform, and the sum of these time intervals (Nishiwaki et al. 2017; Sugawara et al. 2019; Tomoto et al. 2017). Furthermore, the cardio-ankle vascular index (CAVI) was automatically calculated. hbPWV reflects stiffness from the heart to the brachial artery and can serve as a marker of proximal aortic stiffness (Sugawara et al. 2019). baPWV reflects the stiffness of the abdominal aorta and the leg arteries. haPWV reflects the stiffness from the aorta to the ankle, and CAVI is a parameter of haPWV adjusted by BP (Shirai et al. 2011). In our laboratory, the day-to-day coefficients of variation for hbPWV, baPWV, haPWV, and CAVI were 2.4 ± 0.2%, 1.5 ± 0.1%, 1.5 ± 0.1%, and 2.5 ± 0.3%, respectively.
Experiment 2
Arterial stiffness and hemodynamic variables were measured at baseline and at 5 min, 30 min, and 50 min after the first task using the same system. The degrees of change in arterial stiffness measures before and after the first (i.e., from baseline to 5 min after the first task) and second (i.e., from 30 to 50 min after the first task) tasks in both trials were calculated.
Before and during both the first and second tasks, SBP, DBP, and MAP were measured using an automated sphygmomanometer (Tango M2; SunTech Medical Instruments, North Carolina, USA). The BP measurement was carried out twice (approximately, 2 min and 4 min after the beginning of the task) during the task, and the average value was calculated. HR was also measured continuously using the same ECG system. The HR data obtained concurrently with the BP measurement were averaged. Furthermore, after completion of the task, the participants were asked to rate their perceived stress during the task using a standard five-point scale of 0 (not stressful), 1 (somewhat stressful), 2 (stressful), 3 (very stressful), and 4 (very, very stressful) (Callister et al. 1992).
HR was continuously recorded using the same ECG system during the 10-min BS exercise in the EX trial; it was also obtained during the seated rest period in the RE trial. Because of the unstable states at the beginning of the exercise in the EX trial, the HR data for the last 5 min in each trial were averaged. The step rhythm during the same time in the EX trial was recorded and averaged.
To assess mood alterations associated with the 10-min seated rest or BS exercise immediately before and after rest or exercise, the participants rated their perceived level of irritation, confusion, anxiety, and vigor using a 0–10 Likert scale.
Statistics
Data are expressed as means ± SE. The perceived stress levels pertaining to each task were compared between the trials, and the degrees of change in arterial stiffness measures before and after the first and second tasks were compared between the tasks; both comparisons were performed using a paired Student’s t test. Two-way (time × trial) repeated-measures ANOVA with Bonferroni-corrected post hoc testing was performed for arterial stiffness measures and hemodynamic and mood variables. Statistical significance was considered at a P value < 0.05. The statistical analyses were conducted using SPSS version 28.0 (IBM SPSS Japan, Tokyo, Japan).
Results
Experiment 1
In the RE trial, the average HR during seated rest was 71 ± 1 bpm. In the EX trial, the value during the BS exercise was 120 ± 1 bpm, which corresponded to 38.5 ± 0.3% of HRR; the average step rhythm was 114 ± 2 steps/min.
All arterial stiffness measures exhibited a significant decrease for 30 min after the exercise in the EX trial (Fig. 3). Changes in hemodynamic variables are presented in Online Resource 1. HR significantly increased at 10 and 20 min after the exercise in the EX trial and was significantly higher in the EX trial than in the RE trial. SBP and MAP were significantly higher at 10 min after the exercise in the EX trial than in the RE trial.
Measurements of hbPWV (a), baPWV (b), haPWV (c), and CAVI (d) in experiment 1. RE rest, EX exercise, hbPWV heart–brachial pulse wave velocity, baPWV brachial–ankle pulse wave velocity, haPWV heart–ankle pulse wave velocity, CAVI cardio-ankle vascular index. *P < 0.05 vs. baseline. #P < 0.05 vs. the RE trial. Data are expressed as means ± SE
Experiment 2
The average HR value during seated rest was 69 ± 2 bpm in the RE trial. In the EX trial, the HR value during exercise was 120 ± 1 bpm, which corresponded to 38.8 ± 0.4% of HRR; the average value of the step rhythm was 110 ± 2 steps/min.
HR and BP measures increased significantly in both trials in response to the first task, with no significant difference between the trials (Table 1). In both trials, HR and SBP increased significantly in response to the second task, with no significant difference between the trials. DBP and MAP increased significantly in both trials; however, this increase was significantly smaller in the EX trial than in the RE trial. We found no significant difference in the perceived stress level between the trials for both the first task (RE trial: 2.9 ± 0.1 vs. EX trial: 2.8 ± 0.2) and second task (RE trial: 2.7 ± 0.2 vs. EX trial: 2.7 ± 0.2).
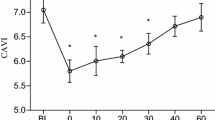
The results of the arterial stiffness are presented in Fig. 4. Both trials resulted in significant elevations in hbPWV, haPWV, and CAVI at 5 min after the first task. The values remained elevated at 30 min after the task in the RE trial, while they returned to baseline levels at this time point in the EX trial. In the RE trial, baPWV increased significantly at 30 min after the task, whereas it did not change significantly in the EX trial over the same period. At 30 min after the task, baPWV, haPWV, and CAVI were significantly lower in the EX trial than in the RE trial. As for the second task (changes from 30 to 50 min after the first task), the variables tended to increase in the RE trial, but this did not reach statistical significance. In the EX trial, hbPWV, haPWV, and CAVI exhibited significant elevations. At 50 min after the task, baPWV and haPWV were significantly lower in the EX trial than in the RE trial. Changes in hemodynamic variables are summarized in Online Resource 2. HR was significantly elevated at 30 min and 50 min after the first task in the EX trial; it was significantly higher than that in the RE trial at 30 min after the task. In the EX trial, the SBP increased significantly at 30 min after the first task, which was significantly higher than that in the RE trial. In both trials, DBP and MAP increased to a similar extent at 5 min after the first task; however, statistical significance was reached only in the RE trial. Compared with the baseline values, DBP and MAP values were significantly higher at 50 min after the first task in the RE trial and at 30 min after the task in the EX trial.
Measurements of hbPWV (a), baPWV (b), haPWV (c), and CAVI (d) in experiment 2. The dashed lines indicate the time point of the first and second tasks. The shaded boxes indicate the duration of seated rest or bench step exercise. hbPWV heart–brachial pulse wave velocity, baPWV brachial–ankle pulse wave velocity, haPWV heart–ankle pulse wave velocity, CAVI cardio-ankle vascular index. *P < 0.05 vs. baseline. †P < 0.05 vs. 30 min after the first task. #P < 0.05 vs. the RE trial. Data are expressed as means ± SE
In the RE trial, ΔhbPWV, ΔhaPWV, and ΔCAVI were significantly lower with the second task than with the first task (Table 2), whereas there were no significant differences in any variables between the tasks in the EX trial.
Irritation decreased significantly in both trials, with no significant difference between the trials (Table 3). No significant change was noted in confusion in either trial, whereas a significant decrease was found in anxiety in the EX trial. Vigor was significantly elevated in the EX trial and was significantly higher than that in the RE trial.
Discussion
The present study aimed to evaluate the effectiveness of BS exercise for ameliorating acute MS-induced arterial stiffening. Our major findings are as follows. In experiment 1, we observed a decrease in arterial stiffness after 10 min of BS exercise. In experiment 2, we found that an elevation in hbPWV, baPWV, haPWV, and CAVI after the first task was completely reversed by performing a 10-min BS exercise. However, hbPWV, haPWV, and CAVI exhibited significant elevations in response to the second task, even among those who had previously exercised. Collectively, these findings suggest that a 10-min BS exercise after acute MS counteracts stress-induced arterial stiffening, but confers only limited protection against the effects of subsequent acute MS.
Although beneficial vascular adaptation has been shown previously for chronic BS exercise training (Ohta et al. 2012), the effect of acute BS exercise on arterial stiffness remains to be investigated. Thus, in experiment 1, we sought to resolve this point and found that a 10-min BS exercise decreased hbPWV, baPWV, haPWV, and CAVI for 30 min. The decrease was observed in all arterial segments measured in this study, suggesting a systemic modulation of arterial stiffness; this finding is supported by previous studies on other exercise modalities (Kingwell et al. 1997; Kume et al. 2021; Okamoto et al. 2018). The present study demonstrates that short-term BS exercise can acutely decrease arterial stiffness and suggests that this exercise has the potential to ameliorate acute MS-induced arterial stiffening.
In experiment 2, the first task caused an elevation in hbPWV, haPWV, and CAVI, which persisted until 30 min after the task in the RE trial; baPWV was also elevated at 30 min after the task. This response pattern is congruent with the findings of our previous study (Kume et al. 2021). The possible physiological reasons for the delayed elevation in baPWV have already been described (Kume et al. 2020, 2021). Specifically, the vasodilatory response and increases in blood flow in the limb vasculature are elicited during acute MS (Carter et al. 2005; Kuipers et al. 2008). Therefore, it is possible that vasodilation and the accompanying increases in blood flow and shear stress in the lower-limb vasculature occurred in the present study, which alleviated arterial stiffening in the leg segment transiently (Heffernan et al. 2007b). This may have led to a delay in the elevation of baPWV. In the same trial, arterial stiffness measures tended to additively increase in response to the second task, but this did not reach statistical significance; indeed, the increases in arterial stiffness measures with the second task were clearly smaller than those with the first task. Endothelial function, a determinant of arterial stiffness (McEniery et al. 2006), is known to be impaired by acute MS (Ghiadoni et al. 2000; Sales et al. 2014; Spieker et al. 2002), and a significant further impairment was evoked upon re-exposure to stress (Sales et al. 2014). The inconsistency in vascular response to re-exposure to acute MS between this study and the previous one (Sales et al. 2014) might be because there was little room for an increase in arterial stiffness in response to this insult, although a more pronounced increase has been reported in response to other stimuli (Heffernan et al. 2007a; Okamoto et al. 2017). Additionally, the difference in participants’ characteristics (young healthy individuals in this study vs. individuals with metabolic syndrome in the previous study) could play a role.
Using cycling as an exercise modality, we have previously demonstrated that acute MS-induced arterial stiffening can be counteracted by incorporating 10 min of exercise thereafter (Kume et al. 2021). In this study, we determined whether such a positive effect could also be obtained by BS exercise. As expected, in the EX trial, exercise completely reversed the elevation in hbPWV, baPWV, haPWV, and CAVI at 30 min after the first task. Notably, the exercise effect was seen in all parameters; this is in agreement with the systemic modulation observed in experiment 1. Our findings reveal for the first time that a 10-min BS exercise after acute MS counteracts arterial stiffening caused by stress. The precise physiological mechanisms of the counteracting effect of BS exercise are currently unclear. In our previous study (Kume et al. 2021), we proposed that the restoration of endothelial function was a major contributor of a similar effect obtained by cycling exercise, based on literature concerning endothelial vasodilatory response to exercise (Goto et al. 2007; Morishima et al. 2019a; Restaino et al. 2015). We speculate that the endothelial mechanism was likely affected similarly in the present study. Some studies have suggested that beneficial mood alternation would lead to a transient amelioration of arterial stiffness or endothelial function (Miller et al. 2006; Sugawara et al. 2010; Vlachopoulos et al. 2009). In this study, an immediate improvement induced by exercise was found in some mood assessment items, which was also likely involved in the observed counteracting effect on arterial stiffness. Further work is needed to clarify the relevant mechanisms.
In the present study, we further examined the effect of a 10-min BS exercise on arterial stiffness response to subsequent acute MS. As a result, although increases in DBP and MAP were attenuated during the second task in the EX trial, a significant elevation was seen in the hbPWV, haPWV, and CAVI after the task. The increases in arterial stiffness measures in the second task were comparable to those of the first task. In experiment 1, the BS exercise induced a lowering of arterial stiffness for 30 min, and the stiffness parameters were measured at 30 min after the exercise ended. Therefore, it is plausible that the observed significant elevation in hbPWV, haPWV, and CAVI was because the MS-induced stiffening overrode the destiffening by exercise and not because of the disappearance of the exercise effect. The lack of elevation in baPWV in response to the second task in the same trial could be explained by the fact that the task-induced increase in this parameter was delayed, making it difficult to detect a change at the predetermined time point (5 min after the task); however, the underlying mechanism is not clear. The present findings suggest that the effect of a 10-min BS exercise on arterial stiffness response to subsequent acute MS may be minor. One possible reason for this may be that the duration of the exercise session in this study was insufficient. We used a 10-min exercise duration, based on our previous study (Kume et al. 2021). This duration was considerably shorter than that employed by Sales et al. (2014) in a study showing the preventive effect of exercise on adverse vascular response to subsequent acute MS. Arterial stiffness can be decreased further by prolonged exercise (compared to a short exercise session) (Kobayashi et al. 2017). If a longer exercise period is used in this setting, the arterial stiffness response to the second task may be offset, but this would result in reduced applicability of this exercise approach in daily life. Morishima et al. (2019a) have reported that resistance exercise induces a transient endothelial dysfunction, which can be restored by a short-term (10-min) cycling exercise after resistance exercise. However, when a 45-min cycling exercise was performed prior to resistance exercise, a sufficient preventive effect was not found (Morishima et al. 2019b). Hence, it may be argued that prior exercise cannot always positively modulate adverse vascular effects of a subsequent stimulus.
Our study provides important evidence showing that a 10-min BS exercise after acute MS is effective to counteract arterial stiffening caused by stress. There has been a dramatic increase in the number of people working from home because of the COVID-19 pandemic (Organisation for Economic Cooperation and Development 2020). A recent study (Fukushima et al. 2021) reported that engaging in working from home resulted in less physical activity and longer sedentary behavior time during work, compared with working at a worksite. BS exercise can be readily performed at home as well as in the office and would thus be a feasible means to protect the vasculature from daily stress. Conversely, we showed that the 10-min BS exercise did not offset an increase in arterial stiffness in response to subsequent acute MS, implying that it may be preferable to engage in brief exercise every time a stressful situation arises. Furthermore, the effectiveness of an exercise as short as 3 min as a countermeasure for adverse physiological consequences has been demonstrated for other types of insults (Dempsey et al. 2016; Moore et al. 2020). Therefore, further work applying BS exercise with a shorter duration (i.e., 3 min) is warranted. We believe that this series of studies will be helpful in formulating a practical and realistic strategy for ameliorating the detrimental vascular effects of acute MS.
There are some limitations to this study. First, only young healthy men were included. Therefore, the study findings may not be generalizable to other populations. Second, although we determined changes in hbPWV (a useful marker of proximal aortic stiffness) (Sugawara et al. 2019), we did not measure carotid–femoral PWV, the standard technique for assessing aortic stiffness.
Conclusion
The present study suggests that a 10-min BS exercise after acute MS can counteract arterial stiffening caused by stress, but the mitigating effect of exercise on subsequent acute MS is limited.
Abbreviations
- ANOVA:
-
Analysis of variance
- baPWV:
-
Brachial–ankle pulse wave velocity
- BP:
-
Blood pressure
- BS:
-
Bench step
- CAVI:
-
Cardio-ankle vascular index
- DBP:
-
Diastolic blood pressure
- ECG:
-
Electrocardiogram
- EX trial:
-
Exercise trial
- haPWV:
-
Heart–ankle pulse wave velocity
- hbPWV:
-
Heart–brachial pulse wave velocity
- HR:
-
Heart rate
- HRR:
-
Heart rate reserve
- MAP:
-
Mean arterial pressure
- MS:
-
Mental stress
- PWV:
-
Pulse wave velocity
- RE trial:
-
Rest trial
- SBP:
-
Systolic blood pressure
References
Callister R, Suwarno NO, Seals DR (1992) Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol 454:373–387. https://doi.org/10.1113/jphysiol.1992.sp019269
Carter JR, Kupiers NT, Ray CA, Ray CA (2005) Neurovascular responses to mental stress. J Physiol 564:321–327. https://doi.org/10.1113/jphysiol.2004.079665
Chida Y, Steptoe A (2010) Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 55:1026–1032. https://doi.org/10.1161/Hypertensionaha.109.146621
Dempsey PC, Sacre JW, Larsen RN, Straznicky NE, Sethi P, Cohen ND, Cerin E, Lambert GW, Owen N, Kingwell BA, Dunstan DW (2016) Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens 34:2376–2382. https://doi.org/10.1097/HJH.0000000000001101
Fukushima N, Machida M, Kikuchi H, Amagasa S, Hayashi T, Odagiri Y, Takamiya T, Inoue S (2021) Associations of working from home with occupational physical activity and sedentary behavior under the COVID-19 pandemic. J Occup Health 63:e12212. https://doi.org/10.1002/1348-9585.12212
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43:1334–1359. https://doi.org/10.1249/MSS.0b013e318213fefb
Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O’Connor G, Betteridge J, Klein N, Steptoe A, Deanfield JE (2000) Mental stress induces transient endothelial dysfunction in humans. Circulation 102:2473–2478. https://doi.org/10.1161/01.cir.102.20.2473
Goto C, Nishioka K, Umemura T, Jitsuiki D, Sakagutchi A, Kawamura M, Chayama K, Yoshizumi M, Higashi Y (2007) Acute moderate-intensity exercise induces vasodilation through an increase in nitric oxide bioavailiability in humans. Am J Hypertens 20:825–830. https://doi.org/10.1016/j.amjhyper.2007.02.014
Hamer M, Taylor A, Steptoe A (2006) The effect of acute aerobic exercise on stress related blood pressure responses: a systematic review and meta-analysis. Biol Psychol 71:183–190. https://doi.org/10.1016/j.biopsycho.2005.04.004
Heffernan KS, Collier SR, Kelly EE, Jae SY, Fernhall B (2007a) Arterial stiffness and baroreflex sensitivity following bouts of aerobic and resistance exercise. Int J Sports Med 28:197–203. https://doi.org/10.1055/s-2006-924290
Heffernan KS, Edwards DG, Rossow L, Jae SY, Fernhall B (2007b) External mechanical compression reduces regional arterial stiffness. Eur J Appl Physiol 101:735–741. https://doi.org/10.1007/s00421-007-0550-4
Kingwell BA, Berry KL, Cameron JD, Jennings GL, Dart AM (1997) Arterial compliance increases after moderate-intensity cycling. Am J Physiol 273:H2186–H2191. https://doi.org/10.1152/ajpheart.1997.273.5.H2186
Kivimäki M, Steptoe A (2018) Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol 15:215–229. https://doi.org/10.1038/nrcardio.2017.189
Kobayashi R, Hatakeyama H, Hashimoto Y, Okamoto T (2017) Acute effects of different aerobic exercise duration on pulse wave velocity in healthy young men. J Sports Med Phys Fitness 57:1695–1701. https://doi.org/10.23736/S0022-4707.16.06894-8
Kuipers NT, Sauder CL, Carter JR, Ray CA (2008) Neurovascular responses to mental stress in the supine and upright postures. J Appl Physiol 104:1129–1136. https://doi.org/10.1152/japplphysiol.01285.2007
Kume D, Nishiwaki M, Hotta N, Endoh H (2020) Impact of acute mental stress on segmental arterial stiffness. Eur J Appl Physiol 120:2247–2257. https://doi.org/10.1007/s00421-020-04448-9
Kume D, Nishiwaki M, Hotta N, Endoh H (2021) Acute mental stress-caused arterial stiffening can be counteracted by brief aerobic exercise. Eur J Appl Physiol 121:1359–1366. https://doi.org/10.1007/s00421-021-04618-3
Lima BB, Hammadah M, Kim JH, Uphoff I, Shah A, Levantsevych O, Almuwaqqat Z, Moazzami K, Sullivan S, Ward L, Kutner M, Ko YA, Sheps DS, Bremner JD, Quyyumi AA, Vaccarino V (2019) Association of transient endothelial dysfunction induced by mental stress with major adverse cardiovascular events in men and women with coronary artery disease. JAMA Cardiol 4:988–996. https://doi.org/10.1001/jamacardio.2019.3252
McEniery CM, Wallace S, Mackenzie IS, McDonnell B, DE Yasmin N, Cockcroft JR, Wilkinson IB (2006) Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 48:602–608. https://doi.org/10.1161/01.HYP.0000239206.64270.5f
Miller M, Mangano C, Park Y, Goel R, Plotnick GD, Vogel RA (2006) Impact of cinematic viewing on endothelial function. Heart 92:261–262. https://doi.org/10.1136/hrt.2005.061424
Moore J, Salmons H, Vinoskey C, Kressler J (2020) A single one-minute, comfortable paced, stair-climbing bout reduces postprandial glucose following a mixed meal. Nutr Metab Cardiovasc Dis 30:1967–1972. https://doi.org/10.1016/j.numecd.2020.06.020
Morishima T, Iemitsu M, Ochi E (2019a) Short-term cycling restores endothelial dysfunction after resistance exercise. Scand J Med Sci Sports 29:1115–1120. https://doi.org/10.1111/sms.13434
Morishima T, Toyoda M, Ochi E (2019b) Prior cycling exercise does not prevent endothelial dysfunction after resistance exercise. Eur J Appl Physiol 119:1663–1669. https://doi.org/10.1007/s00421-019-04154-1
Nishiwaki M, Takahara K, Matsumoto N (2017) Arterial stiffness in young adult swimmers. Eur J Appl Physiol 117:131–138. https://doi.org/10.1007/s00421-016-3505-9
Ohta M, Hirao N, Mori Y, Takigami C, Eguchi M, Tanaka H, Ikeda M, Yamato H (2012) Effects of bench step exercise on arterial stiffness in post-menopausal women: contribution of IGF-1 bioactivity and nitric oxide production. Growth Horm IGF Res 22:36–41. https://doi.org/10.1016/j.ghir.2011.12.004
Okamoto T, Kobayashi R, Sakamaki-Sunaga M (2017) Effect of resistance exercise on arterial stiffness during the follicular and luteal phases of the menstrual cycle. Int J Sports Med 38:347–352. https://doi.org/10.1055/s-0043-101377
Okamoto T, Min SK, Sakamaki-Sunaga M (2018) Acute effect of interval walking on arterial stiffness in healthy young adults. Int J Sports Med 39:495–501. https://doi.org/10.1055/a-0608-4476
Organisation for Economic Cooperation and Development (2020) Productivity gains from teleworking in the post COVID-19 era: How can public policies make it happen? https://www.oecd.org/coronavirus/policy-responses/productivity-gains-from-teleworking-in-the-post-covid-19-era-a5d52e99/. Updated 7 December 2020. Accessed 23 Dec 2021
Poitras VJ, Pyke KE (2013) The impact of acute mental stress on vascular endothelial function: evidence, mechanisms and importance. Int J Psychophysiol 88:124–135. https://doi.org/10.1016/j.ijpsycho.2013.03.019
Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J (2015) Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol 100:829–838. https://doi.org/10.1113/ep085238
Sales AR, Fernandes IA, Rocha NG, Costa LS, Rocha HN, Mattos JD, Vianna LC, Silva BM, Nóbrega AC (2014) Aerobic exercise acutely prevents the endothelial dysfunction induced by mental stress among subjects with metabolic syndrome: the role of shear rate. Am J Physiol Heart Circ Physiol 306:H963–H971. https://doi.org/10.1152/ajpheart.00811.2013
Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, Miyashita Y, Saiki A, Takahashi M, Suzuki K, Takata M (2011) Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb 18:924–938. https://doi.org/10.5551/jat.7716
Spieker LE, Hürlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Lüscher TF, Noll G (2002) Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation 105:2817–2820. https://doi.org/10.1161/01.cir.0000021598.15895.34
Sugawara J, Tarumi T, Tanaka H (2010) Effect of mirthful laughter on vascular function. Am J Cardiol 106:856–859. https://doi.org/10.1016/j.amjcard.2010.05.011
Sugawara J, Tomoto T, Tanaka H (2019) Heart-to-brachium pulse wave velocity as a measure of proximal aortic stiffness: MRI and longitudinal studies. Am J Hypertens 32:146–154. https://doi.org/10.1093/ajh/hpy166
Tomoto T, Maeda S, Sugawara J (2017) Relation between arterial stiffness and aerobic capacity: Importance of proximal aortic stiffness. Eur J Sport Sci 17:571–575. https://doi.org/10.1080/17461391.2016.1277787
Vlachopoulos C, Kosmopoulou F, Alexopoulos N, Ioakeimidis N, Siasos G, Stefanadis C (2006) Acute mental stress has a prolonged unfavorable effect on arterial stiffness and wave reflections. Psychosom Med 68:231–237. https://doi.org/10.1097/01.psy.0000203171.33348.72
Vlachopoulos C, Xaplanteris P, Alexopoulos N, Aznaouridis K, Vasiliadou C, Baou K, Stefanadi E, Stefanadis C (2009) Divergent effects of laughter and mental stress on arterial stiffness and central hemodynamics. Psychosom Med 71:446–453. https://doi.org/10.1097/PSY.0b013e318198dcd4
Zhou Z, He Z, Yuan M, Yin Z, Dang X, Zhu J, Zhu W (2015) Longer rest intervals do not attenuate the superior effects of accumulated exercise on arterial stiffness. Eur J Appl Physiol 115:2149–2157. https://doi.org/10.1007/s00421-015-3195-8
Acknowledgements
The authors sincerely thank the study participants for their cooperation. We also acknowledge Dr. T. Morishima (Chukyo University) for an insightful discussion.
Funding
This work was supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, and Science (#20K11480 to DK).
Author information
Authors and Affiliations
Contributions
DK conceived and designed the study. DK performed the experiments. DK and MN analyzed the data. DK, MN, RT, and NH interpreted the results of the experiments. DK, MN, RT, and NH drafted the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Communicated by Massimo Pagani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kume, D., Nishiwaki, M., Takahara, R. et al. The effectiveness of bench step exercise for ameliorating acute mental stress-induced arterial stiffening. Eur J Appl Physiol 122, 1875–1884 (2022). https://doi.org/10.1007/s00421-022-04962-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-04962-y