Abstract
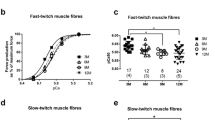
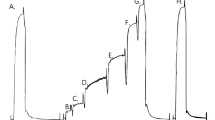
The postnatal growth of rats involves a developmental phase (0 to ∼3 weeks), a rapid growth phase (∼3 to ∼10 weeks), and a slower maturation phase (∼10 weeks+). In this study, we investigated the age-related changes in excitation–contraction (E–C) coupling characteristics of mammalian skeletal muscle, during rapid growth (4–10 weeks) and maturation (10–21 weeks) phases, using single, mechanically skinned fibres from rat extensor digitorum longus (EDL) muscle. Fibres from rats aged 4 and 8 weeks produced lower maximum T-system depolarization-induced force responses and fewer T-system depolarization-induced force responses to 75% run-down than those produced by fibres from rats aged 10 weeks and older. The sensitivity of the contractile apparatus to Ca2+ in fibres from 4-week rats was significantly higher than that in fibres from 10-week rats; however, the maximum Ca2+-activated force per skinned fibre cross-sectional area (specific force) developed by fibres from 4-week rats was on average ∼44% lower than the values obtained for all the other age groups. In agreement with the age difference in specific force, the MHC content of EDL muscles from 4-week rats was ∼29% lower than that of 10-week rats. Thus, mechanically skinned fibres from rats undergoing rapid growth are less responsive to T-system depolarization and maximal Ca2+ activation than fibres from rats at the later stage of maturation or adult rats. These results suggest that during the rapid growth phase in rats, the structure and function of elements involved in E–C coupling in fast-twitch skeletal muscle continue to undergo significant changes.





Similar content being viewed by others
References
Adams GR, McCue SA, Zeng M, Baldwin KM (1999) Time course of myosin heavy chain transitions in neonatal rats: importance of innervation and thyroid state. Am J Physiol 276:R954–R961
Albis A, Couteaux R, Janmot C, Roulet A (1989) Specific programs of myosin expression in the postnatal development of rat muscles. Eur J Biochem 183:583–590
Beam KG, Knudson CM (1988) Effect of postnatal development on calcium currents and slow charge movement in mammalian skeletal muscle. J Gen Physiol 91:799–815
Belcastro AN (1987) Myofibril and sarcoplasmic reticulum changes during muscle development: activity vs inactivity. Int J Biochem 19:945–948
Bortolotto SK, Stephenson DG, Stephenson GM (1999) Fibre type populations and Ca2+-activation properties of single fibres in soleus muscles from SHR and WKY rats. Am J Physiol 276:C628–C637
Bortolotto SK, Cellini M, Stephenson DG, Stephenson GM (2000) MHC isoform composition and Ca(2+)- or Sr(2+)-activation properties of rat skeletal muscle fibres. Am J Physiol Cell Physiol 279:C1564–C1577
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 72:248–254
Chaplin ER, Nell GW, Walker SM (1970) Excitation–contraction latencies in postnatal rat skeletal muscle fibres. Exp Neurol 29:142–151
Close R (1964) Dynamic properties of fast and slow skeletal muscles of the rat during development. J Physiol 173:74–95
Conte Camerino D, De Luca A, Mambrini M, Vrbova G (1989) Membrane ionic conductances in normal and denervated skeletal muscle of the rat during development. Pflugers Arch 413:568–570
Di Maso NA, Caiozzo VJ, Baldwin KM (2000) Single-fibre myosin heavy chain polymorphism during postnatal development: modulation by hypothyroidism. Am J Physiol 278:R1099–R1106
Drachman DB, Johnston DM (1973) Development of a mammalian fast muscle: dynamic and biochemical properties correlated. J Physiol 234:29–42
Edge MB (1970) Development of apposed sarcoplasmic reticulum at the T system and sarcolemma and the change in orientation of triads in rat skeletal muscle. Dev Biol 23:634–650
Ellender G, Feik SA, Carach BJ (1988) Periosteal structure and development in a rat caudal vertebra. J Anat 158:173–187
Endo M, Iino M (1980) Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J Muscle Res Cell Motil 1:89–100
Fink RH, Stephenson DG, Williams DA (1986) Potassium and ionic strength effects on the isometric force of skinned twitch muscle fibres of the rat and toad. J Physiol 370:317–337
Fink RH, Stephenson DG (1987) Ca2+-movements in muscle modulated by the state of K+-channels in the sarcoplasmic reticulum membranes. Pflugers Arch 409:374–380
Fraysse B, Rouaud T, Millour M, Fontaine-Perus J, Gardahaut MF, Levitsky DO (2001) Expression of the Na+/Ca2+ exchanger in skeletal muscle. Am J Phsyiol 280:C146–C154
Geiger PC, Cody MJ, Macken RL, Sieck GC (2000) Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibres. J Appl Physiol 89:695–703
Goodman CA, Stephenson GM (2000) Glycogen stability and glycogen phosphorylase activities in isolated skeletal muscles from rat and toad. J Muscle Res Cell Motil 21:655–662
Goodman CA, Patterson M, Cellini M, Stephenson GMM (2000) MHC isoform expression and E–C coupling in mechanically skinned fibres of the rat. The Physiologist 43(4):357
Goodman CA, Patterson M, Stephenson GMM (2003) MHC-based fibre type and E–C coupling characteristics in mechanically skinned muscle fibres of the rat. Am J Physiol 284:C1448–C1459
Goodman C, Blazev R, Stephenson GMM (2005) Glycogen content and contractile responsiveness to T-system depolarisation in skinned muscle fibres of the rat. Clin Exp Pharmacol Physiol 32:749–756
Hansen O (2001) The a1 isoform of the Na+, K+-ATPase in rat soleus and extensor digitorum longus. Acta Physiol Scand 173:335–341
Hazlewood CF, Nichols BL (1967) In vitro investigation of resting muscle membrane potential in preweanling and weanling rat. Nature 213:935–936
Kjeldsen K, Norgaard A, Clausen T (1984) The age-dependent changes in the number of 3H-ouabain binding sites in mammalian skeletal muscle. Pflugers Arch 402:100–108
Kyselovic J, Leddy JJ, Ray A, Wigle J, Tuana BS (1994) Temporal differences in the induction of dihydropyridine receptor subunits and ryanodine receptors during skeletal muscle development. J Biol Chem 269:21770–21777
Lamb GD, Stephenson DG (1990) Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. J Physiol 423:495–517
Lamb GD, Stephenson DG (1994) Effects of intracellular pH and [Mg2+] on excitation–contraction coupling in skeletal muscle fibres of the rat. J Physiol 478:331–339
Lamb GD, Junankar PR, Stephenson DG (1995) Raised intracellular [Ca2+] abolishes excitation–contraction coupling in skeletal muscle fibres of rat and toad. J Physiol 489:349–362
Lamb GD, Stephenson DG (1996) Effects of FK506 and rapamycin on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol 494:569–576
Lamb GD (2002) Excitation–contraction coupling and fatigue mechanisms in skeletal muscle: studies with mechanically skinned fibres. J Muscle Res Cell Motil 23:81–91
Lamb GD (2002) Voltage sensor control of Ca2+ release in skeletal muscle: insights from skinned fibres. Front Biosci 7:d834–d842
Lamb GD, Cellini MA, Stephenson DG (2001) Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J Physiol 531:715–728
Layman DK, Hegarty PVJ, Swan PB (1980) Comparison of morphological and biochemical parameters of growth in rat skeletal muscles. J Anat 130:159–171
Lodder MAN, de Hann A, Lind A, Sargeant AJ (1993) Changes in morphological and functional characteristics of male rat EDL muscle during growth. J Muscle Res Cell Motil 14:47–53
Lodder MAN, de Hann A, Sargeant AJ (1994) Effect of growth on efficiency and fatigue in extensor digitorum longus muscle of the rat. Eur J Appl Physiol 69:429–434
Patterson MF, Stephenson GM, Stephenson DG (2006) Denervation produces different single fiber phenotypes in fast- and slow-twitch hindlimb muscles of the rat. Am J Physiol Cell Physiol 291(3):C518–C528
Pereon Y, Louboutin JP, Noireaud J (1993) Contractile responses in rat extensor digitorum longus muscles at different times of postnatal development. J Comp Physiol [B] 163:203–211
Plant DR, Lynch GS (2002) Excitation–contraction coupling and sarcoplasmic reticulum function in mechanically skinned fibres from fast skeletal muscles of aged mice. J Physiol 543:169–176
Plant DR, Lynch GS, Williams DA (2002) Hydrogen peroxide increases depolarization-induced contraction of mechanically skinned slow twitch fibres from rat skeletal muscles. J Physiol 539:883–891
Posterino GS (2001) “Current” advances in mechanically skinned skeletal muscle fibres. Clin Exp Pharmacol Physiol 28:668–674
Stephenson DG, Williams DA (1981) Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol 317:281–302
Stephenson DG, Nguyen LT, Stephenson GM (1999) Glycogen content and excitation-contraction coupling in mechanically skinned muscle fibres of the cane toad. J Physiol 519:177–187
Tamaki T, Uchiyama S (1995) Absolute and relative growth of rat skeletal muscle. Physiol Behav 57:913–919
Yamashita S, McGrath KF, Yuki A, Tamaki H, Kasuga N, Takekura H (2007) Assembly of transverse tubule architecture in the middle and myotendinous junctional regions in developing rat skeletal muscle fibres. J Muscle Res cell Motil 28:141–151
Acknowledgements
This work was supported by the National Health and Medical Research Council (Australia) and the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Goodman and Blazev contributed equally to the work presented in, and the preparation of, this manuscript.
Rights and permissions
About this article
Cite this article
Goodman, C.A., Blazev, R., Kemp, J. et al. E–C coupling and contractile characteristics of mechanically skinned single fibres from young rats during rapid growth and maturation. Pflugers Arch - Eur J Physiol 456, 1217–1228 (2008). https://doi.org/10.1007/s00424-008-0474-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-008-0474-9