Abstract
2-Oxoglutarate or α-ketoglutarate (αKG) is a substrate of HIF prolyl hydroxylases 1–3 that decrease cellular levels of the hypoxia-inducible factor 1α (HIF-1α) in the presence of oxygen. αKG analogs are applied to stabilize HIF-1α even in the presence of oxygen and thus provide a novel therapeutic option in treating kidney diseases. In the kidneys, the organic anion transporters 1 and 3 (OAT1 and OAT3, respectively) in cooperation with the sodium-dependent dicarboxylate transporter 3 (NaDC3) and the OAT4 might be responsible for the uptake of αKG analogs into and the efflux out of the tubular cells. Using the radiolabelled substrates p-aminohippurate (PAH, OAT1), estrone-3-sulfate (ES; OAT3, OAT4), and succinate (NaDC3), N-oxalylglycine (NOG), dimethyloxalyl glycine (DMOG), 2,4-diethylpyridine dicarboxylate (2,4-DPD), and pyridine-2,4-dicarboxylic acid (PDCA) were tested in cis-inhibition and trans-stimulation experiments. None of these αKG analogs interacted with NaDC3. 2,4-DPD and PDCA inhibited ES uptake by OAT3 moderately. NOG, 2,4-DPD and PDCA, but not DMOG, inhibited PAH uptake by OAT1 significantly. trans-Stimulation experiments and experiments demonstrating stabilization of HIF-1α revealed that NOG and PDCA, but not 2,4-DPD, are translocated by OAT1. All compounds trans-stimulated ES uptake by OAT4, but only PDCA stabilized HIF-1α. The data suggest that OAT1 is involved in the uptake of NOG and PDCA across the basolateral membrane of proximal tubule cells, whereas OAT4 may release these compounds into the primary urine.
Similar content being viewed by others
Introduction
Hypoxia-inducible factors (HIFs) orchestrate the genomic response to hypoxia in a variety of cells. Numerous HIF target genes have been identified, their products being involved in central cellular mechanisms such as glycolysis, angiogenesis, matrix deposition, and erythropoiesis [for review, see 8, 10, 23]. HIFs are α, ß heterodimers. Whereas the ß-subunit of HIF is constitutively present, HIF-α-subunits [23, 25] are regulated by oxygen levels. Under normoxic conditions, HIF-1α is hydroxylated at prolines 564 and 402 by three functional HIF prolyl hydroxylase domains (PHDs [7, 14, 15]). Hydroxylation of these prolines mediates recognition of HIF by the von Hippel–Lindau tumor suppressor protein as part of an E3 ubiquitin ligase, resulting in ubiquitylation of HIF-1α and its degradation in the proteasome [19]. Moreover, the C-terminus of the HIF-1α subunit is hydroxylated by the factor inhibiting HIF (FIH1) in the presence of oxygen which blocks interaction of HIF with the transcriptional coactivator p300 and may serve as a final transcriptional control of HIF target genes under normoxic conditions [18]. In contrast, under hypoxia, the dioxygen-dependent hydroxylases are not active, and the unmodified HIF-1α proteins become transcriptionally active by heterodimerization with HIF-ß.
PHDs belong to the Fe(II) and 2-oxoglutarate (α-ketoglutarate (αKG))-dependent dioxygenase family, which use oxygen and αKG as substrates and contain Fe2+ in their catalytic center. αKG is used as an electron donor and is consequently oxidized to succinate which, in turn, is a physiological inhibitor of the PHDs. Analogs of αKG and citric acid cycle intermediates were developed as potential drugs to inhibit the PHDs and thereby allow the function of HIF even in the presence of oxygen in cell culture [17, 20] as well as in animal studies of acute renal failure [3] and chronic kidney disease [22, 26].
In humans, acute hypoxic kidney injury is associated with extensive proximal tubular necrosis with preservation of glomeruli and distal nephron segments [12], indicating that proximal tubular cells are particularly vulnerable to oxygen deprivation because of their high metabolic activity. An increase in HIF or a decrease in PHD activity would be a therapeutic option to attenuate or even prevent injury of proximal tubule cells under hypoxic conditions. In most in vitro studies, dimethyloxalyl glycine (DMOG) was applied which competes for binding with αKG to the catalytic site of the PHDs, thereby protecting the cells from undergoing apoptosis. Besides DMOG, N-oxalylglycine (NOG), pyridine-2,4-dicarboxylic acid (PDCA), and 2,4-diethylpyridine dicarboxylate (2,4-DPD) are available as PHD inhibitors (Fig. 1). NOG and PDCA are negatively charged and may not be able to enter cells by simple diffusion. If, however, proximal tubule cells possess transporters accepting negatively charged hydrophilic αKG analogs as substrates, these compounds could offer a promising therapeutic approach to selectively reduce kidney injury. The basolateral membrane of proximal tubule cells is equipped with an array of transporters; among them, the organic anion transporters (OATs [for review 1, 2, 5]) are characterized by broad substrate specificities. Substrates for OATs are strikingly chemically diverse, and identified classes include antineoplastics, antibiotics, antihypertensives, non-steroidal anti-inflammatory drugs, biogenic amine metabolites, steroid hormones and their metabolites, and several toxins. Two OATs, OAT1 and OAT3, are located at the basolateral membrane [21] and one, OAT4, at the luminal membrane of proximal tubule cells [6]. All these transporters operate as anion exchangers, i.e., they couple the uptake of an organic anion into the cell to the release of another organic anion from the cell [for review 1, 2, 5]. OAT1 and OAT3 utilize the existing intracellular > extracellular gradient for αKG to drive uphill uptake of organic anions against the inside negative membrane potential. This αKG gradient is established by the sodium-dependent dicarboxylate transporter 3 (NaDC3). Whereas OAT1 and OAT3 accept dicarboxylates from the extracellular as well as from the intracellular side, OAT4 appears to be an asymmetric transporter by accepting dicarboxylates or compounds with dicarboxylate-like structures only from the intracellular side of the transporter [11].
Since the above-mentioned transporters interact with dicarboxylates, we hypothesized that, in proximal tubule cells, PHD-inhibiting αKG analogs are taken up from the interstitial space across the basolateral membrane either by OAT1, OAT3, or NaDC3, and are released into the urine by OAT4. This hypothesis is based on our finding that αKG, succinate, glutarate, and fumarate are high-affinity substrates for these transporters [16], and some of these PHD inhibitors show structural similarities to αKG and glutarate (Fig. 1).
Materials and methods
Transfections
Human OAT1 and OAT3 (GeneBank accession numbers: AF057039 and AF097491) were obtained from Resource Center for Genome Research (Berlin, Germany). Human OAT4 (GeneBank accession number: AL514126) was from Invitrogen (Groningen, The Netherlands). Human NaDC3 possesses the GeneBank accession no. AF154121. The stably transfected human epithelial kidney cells T-REX™-HEK293-OAT1, -HEK293-OAT3, -HEK293-OAT4, and -HEK293-hNaDC3 were established using the Flp-In™ expression system (Invitrogen) according to the manufacturer's instructions. Stably transfected HEK293 cells were grown in flasks coated with 0.1 mg/ml poly-l-lysine in high-glucose DMEM medium (Invitrogen) supplemented with 10 % fetal calf serum (EU approved origin, Gibco/Invitrogen), 1 % penicillin/streptomycin, and blasticidine (5 mg/l; all antibiotics from Sigma, Taufkirchen, Germany). Control cells were transfected with the vector alone. Cultures were maintained in humidified atmosphere that contained 5 % CO2 at 37 °C.
Solutions
A standard mammalian Ringer solution (MRi) was used for the uptake experiments. MRi contained (in millimolars): 130 NaCl, 4 KCl, 1 CaCl2, 1 MgSO4, 1 NaH2PO4, 20 HEPES, and 18 glucose at pH 7.4. Sodium-free conditions were obtained by equimolar substitution of NaCl by tetraethylammonium chloride (TEACl). Test substances for cis-inhibiton or trans-stimulation experiments were succinate, glutarate, α-ketoglutarate (αKG), N-oxalylglycine (NOG), 2,4-diethylpyridine dicarboxylate (2,4-DPD), dimethyloxalyl glycine (DMOG), and pyridine-2,4-dicarboxylic acid (PDCA). Chemicals of analytical grade for the uptake experiments and also for the immunoblots (see below) were either from Sigma or from Applichem (Darmstadt, Germany). The αKG analogs were obtained from Alexis (Alexis/Axxora, Lörrach, Germany).
Tracer uptake experiments
HEK293-OAT1-, -OAT3-, -OAT4-, -NaDC3-, and vector-transfected cells were harvested and plated into 24-well plastic dishes (Sarstedt, Nürmbrecht, Germany) at a density of 2 × 105 cells/well. For protein quantification, a separate plate was used, seeded with the same cell density per well. The protein amount was determined in parallel from six wells for each cell line in nine independent experiments using a photometric method described by Bradford [4]. Transport assays were performed 72 h after seeding the cells in MRi. At the end of culture, cells were washed twice with 0.5 ml MRi and incubated for 5 min in MRi that contained 1.2 μM [3 H]p-aminohippuric acid (3 H-PAH, 4.35 Ci/mmol; Perkin Elmer, Rodgau, Germany) for OAT1-, 10 or 20 nM [3 H]estrone sulfate (3 H-ES, 57.3 Ci/mmol; Perkin Elmer) for OAT3- and OAT4-, or 1 μM [14 C]succinate (14 C-succinate, 58 mCi/mmol; Perkin Elmer) for NaDC3-transfected HEK293 cells. IC50 values for OAT1 were performed with 10 μM total PAH. Uptake was terminated by removal of the radioactive medium and immediate 3 1 ml washes with ice-cold MRi. The cells were dissolved in 0.5 ml 1 N NaOH by gently shaking for 120 min followed by neutralization with 0.5 ml 1 N HCl. The 3 H-or 14 C-content was determined by liquid scintillation counting (Tricarb 2900TR, Perkin Elmer).
For trans-stimulation experiments, either OAT1- or OAT4-transfected HEK293 cells, were preincubated for 2 h in MRi containing either 100 μM DMOG, NOG, 2,4-DPD, or PDCA. Thereafter, the respective cell line was washed two times in MRi and the uptake of radiolabelled PAH (OAT1) or ES (OAT4), respectively, was performed as described above.
Immunoblots
Anti-HIF-1α immunoblots were performed using whole cell extracts taken from OAT1-, OAT4-, or vector-transfected HEK293 cells grown on 10-cm dishes (approx. 3 × 106 cells/dish) to subconfluency. Cells were incubated for 4 h in high glucose DMEM medium (Invitrogen) supplemented with 10 % fetal calf serum (EU approved origin, GIBCO/Invitrogen) containing in addition either 100 μM dipyridil (DP), 1 mM DMOG, 500 μM NOG, 500 μM 2,4-DPD, or 100 μM PDCA in the presence and absence of 1 mM probenecid. Afterwards, cells were lysed in 6.65 M urea, 10 % glycerol, 1 % sodium dodecylsulfate 10 mM TRIS/HCl, 5 mM dithiotreitol, and protease inhibitor Complete® (Roche Diagnostics, Mannheim, Germany). Protein (100 μg) was separated on 10 % polyacrylamide gels, transferred onto polyvinylidene difluoride membranes (BioRad, Munich, Germany) and incubated with antibodies against HIF-1α and ß-actin (1:2,000, Cayman Chemical, Ann Arbor, MI). Subsequently, blots were exposed to horseradish peroxidase-conjugated secondary antibodies (Dako, Hamburg, Germany), and signals were visualized by chemiluminescence (Amersham ECL-Plus, GE healthcare, Buckinghamshire, UK).
Statistics
Paired Student's t tests were used to show statistical significant differences for the inhibition of the uptake of either PAH, ES, or succinate by the test substances. Statistical significance was set at p < 0.001. IC50 values for the inhibition of PAH uptake by NOG, 2,4-DPD, and PDCA were calculated using the SigmaPlot10 software (Systat, Point Richmond, CA, USA). All uptake experiments were performed at least in triplicate using cell cultures from consecutive cell culture passages. The immunoblot (Fig. 7) was performed twice with identical results.
Results
Interactions of α-ketoglutarate (αKG) analogs with the human sodium-dependent dicarboxylate transporter 3 (NaDC3)
In three independent experiments, the 5-min uptake of succinate was determined to 547.7 ± 39.3 and 10.1 ± 0.9 pmol/mg protein in HEK293 cells stably transfected with NaDC3 or vector, respectively. Succinate uptake was abolished when all sodium was replaced by TEA (Fig. 2, sodium-free), demonstrating that NaDC3 was functionally expressed in HEK293 cells (Fig. 2). Unlabelled succinate (100 μM) reduced uptake of labelled succinate by 73.4 ± 4.7 %. Whereas application of the αKG analogs NOG and PDCA and the related compounds DMOG and 2,4-DPD (each 100 μM) did not inhibit succinate uptake (Fig. 2), the naturally occurring dicarboxylates glutarate and αKG inhibited succinate uptake by 60.9 ± 0.7 and 46.2 ± 3.9 %, respectively.
Effect of several αKG analogs on succinate uptake in NaDC3- and vector-transfected HEK293 cells. The 5-min succinate uptake was measured in the absence and presence of succinate, αKG, and various αKG analogs (each 0.1 mM). The uptake in the presence of a putative substrate was calculated as the percentage of the uptake measured in the absence of this compound (none) which was set to 100 %. Values are mean ± SEM of 3–4 independent experiments; asterisk p < 0.001 versus “none”
Influence of αKG analogs on OAT3-transfected HEK293 cells
Functional expression of OAT3 was tested by demonstrating inhibition of ES uptake by unlabelled ES and by probenecid (Fig. 3). At a concentration of 100 μM, 2,4-DPD and PDCA inhibited ES uptake by 31.3 ± 8.3 % and 29.7 ± 5.7 %, respectively. NOG and DMOG exhibited no effect on ES uptake. In vector-transfected cells, ES uptake was similar under all experimental conditions and less than 15 % of the uptake observed in OAT3-transfected HEK293 cells.
Effect of several αKG analogs on ES uptake in OAT3- and vector-transfected HEK293 cells. The 5-min ES uptake was measured in the absence (none) and presence of various compounds. The concentration of the inhibitor used was 100 μM. Values are mean ± SEM of 3–4 independent experiments; asterisk p < 0.001 versus “none”
Influence of αKG analogs on OAT1-transfected HEK293 cells
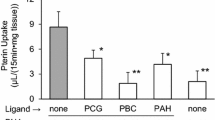
The 5 min uptake of PAH, the prototypical substrate of OAT1, was 91.1 ± 11.7 and 3.1 ± 0.4 pmol/mg protein in OAT1- and vector-transfected cells, respectively. Unlabelled PAH and probenecid evoked a substantial inhibition on PAH uptake, indicating successful transfection of the HEK293 cells with OAT1. From the four compounds tested, NOG, 2,4-DPD, and PDCA at a concentration of 100-μM inhibited uptake of PAH significantly in OAT1-transfected HEK293 cells. DMOG was not effective (Fig. 4A). To investigate whether NOG, 2,4-DPD, and PDCA are not only inhibitors of OAT1-mediated PAH uptake but are also substrates of OAT1, trans-stimulation experiments were performed. To this end, OAT1-transfected HEK293 cells were preloaded for 2 h with 2,4-DPD, DMOG, PDCA, and NOG (Fig. 4B). Uptake of PAH was significantly trans-stimulated upon preloading the cells with NOG, DMOG, and PDCA, but not with 2,4-DPD. To further elucidate, which of the three compounds has the highest potency in inhibiting PAH uptake, IC50 values were determined. NOG (Fig. 5A), 2,4-DPD (Fig. 5B), and PDCA (Fig. 5C) showed inhibition of PAH uptake with IC50 values of 79.6 ± 10.2, 60.9 ± 3.0, and 19.1 ± 6.1 μM, respectively.
Effect of several αKG analogs on PAH uptake in OAT1- and vector-transfected HEK293 cells. a Cis-inhibition of PAH uptake by αKG analogs. The 5-min PAH uptake was measured in the absence (none) and presence of various compounds. The concentration of the inhibitor used was 0.1 mM. b Trans-stimulation of PAH uptake in OAT1-transfected HEK293 cells by NOG, DMOG, and PDCA, but not by 2,4-DPD. Cells were preloaded for 2 h in the indicated compounds. Immediately after washing out of the indicated compound, 5-min PAH uptake was measured. The uptake in the presence of any putative substrate was set to 100 %, and the uptake of the respective αKG analog was set in relation. a, b show the summary of three independent experiments where all compounds were tested simultaneously; asterisk p < 0.001 versus “none”
Determination of IC50 for the inhibition of OAT1-mediated PAH uptake by NOG (a), 2,4-DPD (b), and PDCA (c). Plots were obtained by using 10 μM total PAH and increasing NOG (a), 2,4-DPD (b), or PDCA (c) concentrations up to 500 μM at an uptake time of 5 min. Each plot shows mean values of three independent experiments
Substrate specificity of OAT4-transfected HEK293 cells
OAT4 has recently been identified as an asymmetric transporter, accepting dicarboxylates only from the cytosol. Successful transfection with OAT4 was proven by uptake of ES which was 199.6 ± 14.9 in OAT4-transfected and 25.3 ± 3.2 fmol/mg protein in vector-transfected control cells. ES uptake was inhibited by unlabelled ES and probenecid (Fig. 6A). While application of the αKG analogs (100 μM) on OAT4-transfected HEK293 cells (Fig. 6A) showed no effect on ES uptake, preloading the cells with 2,4-DPD, DMOG, NOG, and PDCA for 2 h (Fig. 6B) resulted in significantly increased uptake of ES (trans-stimulation). As an internal control, glutarate was used in these experiments to prove trans-stimulation by a more physiological dicarboxylate.
Effect of several αKG analogs on ES uptake in OAT4- and vector-transfected HEK293 cells. a cis-inhibition and b trans-stimulation experiments. The 5-min uptake of ES uptake was measured in the absence and presence of various PHD inhibitors and related compounds (100 μM) (a). Cells were preloaded for 2 h in the indicated compounds (500 μM in MRi) (b). Immediately after a wash-out of the indicated compound, 5-min ES uptake was measured. Under both conditions, the uptake in the absence of any putative substrate was set to 100 % and the inhibition (a) or stimulation (b) of uptake was set into relation. Each plot shows the summary of three to four independent experiments; asterisk p < 0.001 versus “none”
Stabilization of HIF-1α in OAT1- and OAT4-transfected HEK293 cells
Finally, we tested for HIF-1α stabilization in HEK293 cells stably transfected with OAT1 or vector alone after treatment with PHD inhibitors in the absence and presence of probenecid (Fig. 7). The Fe2+ chelator dipyridyl (DP) was used as a freely permeable positive control. With the exception of 2,4-DPD, application of all PHD inhibitors induced HIF-1α in OAT1-, but not in vector-transfected cells. Stabilization of HIF-1α was prevented by the simultaneous application of probenecid (1 mM) with either NOG or PDCA (0.5 or 0.1 mM, respectively), indicating that NOG and PDCA were translocated by OAT1. In OAT4-transfected cells, a marginal stabilization of HIF-1α was observed by PDCA, and this effect was abolished by probenecid. DMOG stabilized HIF-1α in OAT1- as well as in OAT4- and in vector-transfected cells, and this effect was partially abolished by probenecid.
Stabilization of HIF-1α in OAT1- and OAT4-tansfected HEK293 cells. HIF-1α immunoblot of OAT1- (top), OAT4- (middle), and vector-transfected (base) HEK293 cells after treatment with the chemical HIF inductor dipyridil (DP) as a positive control or the PHD inhibitors DMOG (1 mM), NOG (0.5 mM), 2,4-DPD (0.5 mM), and PDCA (0.1 mM) in the absence and presence of probenecid (1 mM). The concentrations of NOG, 2,4-DPD, and PDCA were chosen three to five times above the IC50 as determined in OAT1-transfected HEK293 cells. Proteins (0.1 mg) were transferred onto polyvinylidine difluoride membranes and incubated with the antibodies. Blots were performed in duplicate, and both trials showed comparable results
Discussion
Stabilization of HIF by carbon monoxide or available PHD inhibitors has been shown to have significant beneficial effects in models of acute ischemic and toxic kidney injury [3, 9, 12, 13, 22, 26, 27]. On the other hand, hypoxia has been shown to promote interstitial fibrosis and support epithelial-to-mesenchymal transition which has been proposed to be a major pathophysiological mechanism leading to progressive chronic kidney disease [10]. Whether PHD inhibitors structurally related to αKG show also unwanted side effects can only be elucidated in clinical studies. Our experiments, however, demonstrated interaction of PDCA, NOG, and 2,4-DPD with OAT1. The IC50 values for inhibition of PAH uptake by these compounds we obtained varied between 19 and 80 μM. These values are in agreement with recently reported IC50 or Ki values for NOG and PDCA against human αKG dioxygenases which are in the range between 0.5 and 40 μM [24].
Data on the uptake of αKG analogs across the plasma membrane of target cells have not been available so far. Whereas uncharged compounds such as DMOG and 2,4-DPD may cross cell membranes by simple diffusion, uptake of the dianionic NOG and PDCA should require interaction with transporters accepting dicarboxylates. To this end, the impact of NOG, DMOG, 2,4-DPD, and PDCA on the human organic anion transporters OAT1, 3, and 4 and on the human sodium-dependent dicarboxylate transporter (NaDC3) was tested. These transporters are expressed either in the basolateral (OAT1, OAT3, NaDC3) or the luminal membrane (OAT4) of proximal tubule cells. To demonstrate an interaction of the PHD inhibitors with the transporters, concentrations similar to those applied in other cell culture studies (0.1–1 mM [17, 20]) were applied. These concentrations were sufficient to stabilize HIF in vitro.
NaDC3 was not sensitive towards all tested αKG analogs, and only the physiological substrates, glutarate, and αKG inhibited succinate uptake. Hence, it is likely that NaDC3 is not involved in the Na+-dependent uptake of αKG analogs into proximal tubular cells. ES uptake in OAT3-transfected HEK293 cells was not (NOG, DMOG) or weakly (2,4-DPD; PDCA) affected, suggesting a limited, if any, role in the uptake of αKG analogs.
DMOG did not inhibit the uptake of PAH in OAT1-transfected HEK293 cells, indicating that the two methyl groups and the absence of negative charges avoid transport by OAT1. Hydroxylation of HIF can be inhibited by DMOG [for review, see 22, 26], and our own results are shown in Fig. 7. It seems that DMOG permeates the cell membrane not only by simple diffusion but also by a probenecid-inhibitable mechanism, as revealed by comparison of the staining in the immunoblots by DMOG in the absence and presence of probenecid (Fig. 7, 1st, 5th row). The nature of the underlying endogenous transporter present in OAT1- and vector-transfected HEK293 cells is unknown. The second uncharged compound tested, 2,4-DPD, inhibited PAH uptake by OAT1 with an IC50 of approx. 60 μM. Since 2,4-DPD was unable to trans-stimulate PAH uptake and also no stabilization of HIF-1α was observed, we suggest that this compound inhibits OAT1 but itself does not enter cells by diffusion nor by OAT1-mediated transport.
The divalent anions, NOG and PDCA, are substrates of OAT1 as they not only decreased PAH uptake in cis-inhibition experiments but also increased it in trans-stimulation experiments. These data do not prove but strongly suggest that OAT1 translocate NOG and PDCA across cell membranes. An indication that NOG and PDCA are substrates of OAT1 is provided by the stabilization of HIF-1α by NOG and PDCA in OAT1- but not in vector-transfected cells. That these compounds use OAT1 for translocation is supported by the observation that stabilization of HIF-1α is prevented by probenecid, an inhibitor of OAT1.
With respect to OAT4, unexpected results were obtained. Whereas in cis-inhibition experiments αKG analogs were not effective on OAT4-transfected HEK293 cells, all compounds were found to trans-stimulate ES uptake. From these compounds, only PDCA stabilized HIF-1α. It appears that PDCA has a weak affinity to OAT4 which was not detected in cis-inhibition experiments. During the 4-h incubation, however, sufficient PDCA entered the OAT4-transfected HEK293 cells to stabilize HIF-1α. The specificity of this effect was evaluated by the simultaneous application of PDCA and probenecid which prevented stabilization of HIF-1α in these cells.
Preloading cells with NOG and 2,4-DPD afforded a trans-stimulation of OAT4-mediated ES uptake, but no stabilization of HIF-1α. Possibly, OAT4 mainly acts as an efflux transporter keeping intracellular concentrations of NOG and 2,4-DPD too low for a measurable HIF-1α stabilization.
In summary, our data indicate that NOG and PDCA may be taken up from the blood into the proximal tubule cells by OAT1 and released into the primary urine by OAT4.
References
Al VW, Gionfriddo MR, Sweet DH (2010) Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos 31:1–71
Anzai N, Kanai Y, Endou H (2006) Organic anion transporter family: current knowledge. J Pharmacol Sci 100:411–426
Bernhardt WM, Campean V, Kany S, Jürgensen J-S, Weidemann A, Warnecke C, Arend M, Klaus S, Günzler V, Amann K, Willam C, Wiesener MS, Eckhardt K-U (2006) Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17:1970–1978
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burckhardt G, Burckhardt BC (2011) In vitro and in vivo evidence for the importance of organic anion transporters (OATs) in drug therapy. In: Fromm MF, Kim RB (eds) Handbook of Exp Pharmacol 201, Drug Transporters. Springer, Heidelberg, pp 29–104
Ekataratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, Kanai Y, Sophasan S, Endou H (2004) Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci 94:297–304
Epstein ACR, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian Y-M, Masson P, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield PH, Ratcliffe PJ (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43–54
Gardner LB, Corn PG (2006) Hypoxic regulation of mRNA expression. Cell Cycle 7:1916–1924
Haase VH (2006) Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol 291:F271–F281
Haase VH (2009) Oxygen regulates epithelial-to-mesenchymal transition: insights into molecular mechanisms and relevance to disease. Kidney Int 76:492–499
Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A (2007) Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol 18:430–439
Heyman SN, Rosenberger C, Rosen S (2010) Experimental ischemia-reperfusion: biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int 77:9–16
Hill P, Shukla D, Tran MGB, Aragones J, Cook HT, Carmeliet P, Maxwell PH (2008) Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 19:39–46
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr (2001) Hifα targeted for VHL-mediated destruction by proline hydroxylation: implication for O2 sensing. Science 292:464–468
Jaakkola P, Mole DR, Tian Y-M, Wilso MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebstreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472
Kaufhold M, Schulz K, Breljak D, Gupta S, Henjakovic M, Krick W, Hagos Y, Sabolic I, Burckhardt BC, Burckhardt G (2011) Differential interaction of dicarboxylates with human sodium-dicarboxylate cotransporter 3 and organic anion transporters 1 and 3. Am J Physiol Renal Physiol 301:F1026–F1034
Koivunen P, Hirsilä RAM, Hassinen IE, Kivirikko KI, Myllyharju J (2007) Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates. J Biol Chem 282:4524–4532
Mahon PC, Hirota K, Semenza GL (2001) FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 15:2675–2686
Maxwell PH, Wiesener MS, Chang G-W, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumor suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:272–275
Mole DR, Schlemminger I, McNeill LA, Hewitson KS, Pugh CW, Ratcliffe PJ, Schofield CJ (2003) 2-Oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg Med Chem Lett 13:2677–2680
Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goo M, Fukatsu A, Ogawa O, Inui K-I (2002) Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol 13:866–874
Nangaku M, Eckhardt K-U (2007) Hypoxia and the HIF system in kidney disease. J Mol Med 85:1325–1330
Radcliffe PJ (2006) Understanding hypoxia signaling in cells—a new therapeutic opportunity? Clin Med 6:573–578
Rose NR, McDonough MA, King ONF, Kawamura A, Schofield CJ (2011) Inhibition of 2-oxoglutarate dependent oxygenases. Chem Soc Rev 40:4364–4397
Semenza GL (2007) Hypoxia-inducible factor (HIF-1) pathway. Sci STKE 2007 cm8
Tanaka T, Nangaku M (2009) Drug discovery for overcoming chronic kidney disease (CKD): prolyl-hydroxylases inhibitors to activate hypoxia-inducible factor (HIF) as a novel therapeutic approach in CKD. J Pharmacol Sci 109:24–31
Weidemann A, Bernhardt WM, Klanke B, Daniel C, Buchholz B, Campean V, Amann K, Warnecke C, Wiesener MS, Eckhardt K-U, Willam C (2008) HIF activation protects from acute kidney injury. J Am Soc Nephrol 19:486–494
Acknowledgments
The authors want to thank S. Petzke for excellent technical assistance. The study was supported by a grant of the German Research Council to B.C.B. (BU998/5-1).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hagos, Y., Schley, G., Schödel, J. et al. α-Ketoglutarate-related inhibitors of HIF prolyl hydroxylases are substrates of renal organic anion transporters 1 (OAT1) and 4 (OAT4). Pflugers Arch - Eur J Physiol 464, 367–374 (2012). https://doi.org/10.1007/s00424-012-1140-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-012-1140-9