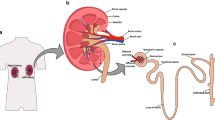
Abstract
The epithelial cells lining the thick ascending limb (TAL) of the loop of Henle perform essential transport processes and secrete uromodulin, the most abundant protein in normal urine. The lack of differentiated cell culture systems has hampered studies of TAL functions. Here, we report a method to generate differentiated primary cultures of TAL cells, developed from microdissected tubules obtained in mouse kidneys. The TAL tubules cultured on permeable filters formed polarized confluent monolayers in ∼12 days. The TAL cells remain differentiated and express functional markers such as uromodulin, NKCC2, and ROMK at the apical membrane. Electrophysiological measurements on primary TAL monolayers showed a lumen-positive transepithelial potential (+9.4 ± 0.8 mV/cm2) and transepithelial resistance similar to that recorded in vivo. The transepithelial potential is abolished by apical bumetanide and in primary cultures obtained from ROMK knockout mice. The processing, maturation and apical secretion of uromodulin by primary TAL cells is identical to that observed in vivo. The primary TAL cells respond appropriately to hypoxia, hypertonicity, and stimulation by desmopressin, and they can be transfected. The establishment of this primary culture system will allow the investigation of TAL cells obtained from genetically modified mouse models, providing a critical tool for understanding the role of that segment in health and disease.







Similar content being viewed by others
References
Allen ML, Nakao A, Sonnenburg WK et al (1988) Immunodissection of cortical and medullary thick ascending limb cells from rabbit kidney. Am J Physiol 255:F704–F710
Allen F, Tisher CC (1976) Morphology of the ascending thick limb of Henle. Kidney Int 9:8–22
Ares GR, Caceres PS, Ortiz PA (2011) Molecular regulation of NKCC2 in the thick ascending limb. Am J Physiol Ren Physiol 301:F1143–F1159
Bates JM, Raffi HM, Prasadan K et al (2004) Tamm–Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int 65:791–797
Baudouin-Legros M, Bouthier M, Teulon J (1993) [Arginine]vasopressin hydrolyses phosphoinositides in the medullary thick ascending limb of mouse nephron. Pflugers Arch 425:381–389
Bourgeois S, Rossignol P, Grelac F et al (2003) Differentiated thick ascending limb (TAL) cultured cells derived from SV40 transgenic mice express functional apical NHE2 isoform: effect of nitric oxide. Pflugers Arch 446:672–683
Burg MB, Ferraris JD, Dmitrieva NI (2007) Cellular response to hyperosmotic stresses. Physiol Rev 87:1441–1474
Burg M, Green N, Sohraby S, Steele R, Handler J (1982) Differentiated function in cultured epithelia derived from thick ascending limbs. Am J Physiol 242:C229–C233
Chamberlin ME, LeFurgey A, Mandel LJ (1984) Suspension of medullary thick ascending limb tubules from the rabbit kidney. Am J Physiol 247:F955–F964
Chang CT, Hung CC, Tian YC, Yang CW, Wu MS (2007) Cyclosporine reduces paracellin-1 expression and magnesium transport in thick ascending limb cells. Nephrol Dial Transplant 22:1033–1040
Dahan K, Devuyst O, Smaers M et al (2003) A cluster of mutations in the UMOD gene causes familial juvenile hyperuricemic nephropathy with abnormal expression of uromodulin. J Am Soc Nephrol 14:2883–2893
Devuyst O (2008) Salt wasting and blood pressure. Nat Genet 40:495–496
Devuyst O, Christie PT, Courtoy PJ et al (1999) Intra-renal and subcellular distribution of the human chloride channel, CLC-5, reveals a pathophysiological basis for Dent's disease. Hum Mol Genet 8:247–257
Di Stefano A, Jounier S, Wittner M (2001) Evidence supporting a role for KCl cotransporter in the thick ascending limb of Henle's loop. Kidney Int 60:1809–1823
Di Stefano A, Roinel N, de Rouffignac C et al (1993) Transepithelial Ca2+ and Mg2+ transport in the cortical thick ascending limb of Henle's loop of the mouse is a voltage-dependent process. Ren Physiol Biochem 16:157–166
Drugge ED, Carroll MA, McGiff JC (1989) Cells in culture from rabbit medullary thick ascending limb of Henle's loop. Am J Physiol 256:C1070–C1081
Dublineau I, Elalouf JM, Pradelles P et al (1989) Independent desensitization of rat renal thick ascending limbs and collecting ducts to ADH. Am J Physiol 256:F656–F663
Eckardt KU, Bernhardt WM, Weidemann A et al (2005) Role of hypoxia in the pathogenesis of renal disease. Kidney Int 99:S46–S51
Eng B, Mukhopadhyay S, Vio CP et al (2007) Characterization of a long-term rat mTAL cell line. Am J Physiol Ren Physiol 293:F1413–F1422
Eveloff J, Haase W, Kinne R (1980) Separation of renal medullary cells: isolation of cells from the thick ascending limb of Henle's loop. J Cell Biol 87:672–681
Gamba G, Friedman PA (2009) Thick ascending limb: the Na(+):K (+):2Cl (−) co-transporter, NKCC2, and the calcium-sensing receptor, CaSR. Pfluegers Arch 458:61–76
Hebert S (1995) An ATP-regulated inwardly rectifying potassium channel from rat kidney. Kidney Int 48:1010–1016
Hebert SC, Culpepper RM, Andreoli TE (1981) NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol 241:F412–F431
Jans F, Vandenabeele F, Helbert M et al (2000) A simple method for obtaining functionally and morphologically intact primary cultures of the medullary thick ascending limb of Henle's loop (MTAL) from rabbit kidneys. Pflugers Arch 440:643–651
Köttgen A (2010) Genome-wide association studies in nephrology research. Am J Kidney Dis 56:743–758
Kwon MS, Lim SW, Kwon HM (2009) Hypertonic stress in the kidney: a necessary evil. Physiology (Bethesda) 24:186–191
Liu Y, Mo L, Goldfarb DS et al (2010) Progressive renal papillary calcification and ureteral stone formation in mice deficient for Tamm–Horsfall protein. Am J Physiol Ren Physiol 299:F469–F478
Lu M, Wang T, Yan Q et al (2002) Absence of small conductance K+channel (SK) activity in apical membranes of thick ascending limb and cortical collecting duct in ROMK (Bartter's) knockout mice. J Biol Chem 277:37881–37887
Meyer AH, Katona I, Blatow M et al (2002) In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J Neurosci 22:7055–7064
Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR (2004) Ablation of the Tamm–Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Ren Physiol 286:795–802
Mutig K, Kahl T, Saritas T et al (2011) Activation of the bumetanide-sensitive Na+, K+,2Cl− cotransporter (NKCC2) is facilitated by Tamm–Horsfall protein in a chloride-sensitive manner. J Biol Chem 286:30200–30210
Mutig K, Paliege A, Kahl T, Jöns T, Müller-Esterl W, Bachmann S (2007) Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Ren Physiol 293:F1166–F1177
Olsen JV, de Godoy LM, Li G et al (2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol Cell Proteomics 12:2010–2021
Pizzonia JH, Gesek FA, Kennedy SM, Coutermarsh BA, Bacskai BJ, Friedman PA (1991) Immunomagnetic separation, primary culture, and characterization of cortical thick ascending limb plus distal convoluted tubule cells from mouse kidney. In Vitro Cell Dev Biol 27A:409–416
Rampoldi L, Scolari F, Amoroso A et al (2011) The rediscovery of uromodulin (Tamm–Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 80:338–347
Rappsilber J, Ishihama Y, Mann M (2003) Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem 75:663–670
Renigunta A, Renigunta V, Saritas T, Decher N, Mutig K, Waldegger S (2011) Tamm–Horsfall glycoprotein interacts with renal outer medullary potassium channel ROMK2 and regulates its function. J Biol Chem 286:2224–2235
Santambrogio S, Cattaneo A, Bernascone I et al (2008) Urinary uromodulin carries an intact ZP domain generated by a conserved C-terminal proteolytic cleavage. Biochem Biophys Res Commun 370:410–413
Schley G, Klanke B, Schödel J et al (2011) Hypoxia-inducible transcription factors stabilization in the thick ascending limb protects against ischemic acute kidney injury. J Am Soc Nephrol 22:2004–2015
Stiehl DP, Wirthner R, Koditz J et al (2006) Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem 281:23482–23491
Terryn S, Jouret F, Vandenabeele F et al (2007) A primary culture of mouse proximal tubular cells, established on collagen-coated membranes. Am J Physiol Ren Physiol 293:F476–F485
Valentich JD, Stokols MF (1986) An established cell line from mouse kidney medullary thick ascending limb. I. Cell culture techniques, morphology, and antigenic expression. Am J Physiol 251:C299–C311
Wiggins RC (1987) Uromucoid (Tamm–Horsfall glycoprotein) forms different polymeric arrangements on a filter surface under different physicochemical conditions. Clin Chim Acta 162:329–340
Wu MS, Bens M, Cluzeaud F, Vandewalle A (1994) Role of F-actin in the activation of Na(+)-K(+)-Cl− cotransport by forskolin and vasopressin in mouse kidney cultured thick ascending limb cells. J Membr Biol 142:323–336
Acknowledgments
The authors would like to thank Gery Barmettler, Soline Bourgeois, Huguette Debaix, David Hoogewijs and Klaus Marquardt for assistance and helpful suggestions. Prof. Jan Loffing kindly provided the parvalbumin-EGFP mouse. The uromodulin knockout mouse was kindly provided by Prof. X-R. Wu. These studies were supported in part by the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 246539 (Marie Curie) and grant no. 305608 (EURenOmics); an Action de Recherche Concertée (ARC, Communauté Française de Belgique); the FNRS and FRSM; the Inter-University Attraction Pole (IUAP, Belgium Federal Government); the NCCR Kidney. CH program (Swiss National Science Foundation); the Gebert Rüf Stiftung (Project GRS-038/12); and the Swiss National Science Foundation 31003A-125422/1 (to OS) and 310030–146490 (to OD).
Conflict of interest
The authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Glaudemans, B., Terryn, S., Gölz, N. et al. A primary culture system of mouse thick ascending limb cells with preserved function and uromodulin processing. Pflugers Arch - Eur J Physiol 466, 343–356 (2014). https://doi.org/10.1007/s00424-013-1321-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1321-1