Abstract
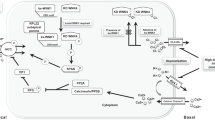
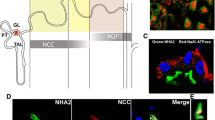
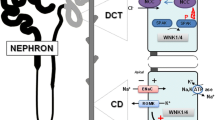
SLC12A3 encodes the thiazide-sensitive sodium chloride cotransporter (NCC), which is primarily expressed in the kidney, but also in intestine and bone. In the kidney, NCC is located in the apical plasma membrane of epithelial cells in the distal convoluted tubule. Although NCC reabsorbs only 5 to 10 % of filtered sodium, it is important for the fine-tuning of renal sodium excretion in response to various hormonal and non-hormonal stimuli. Several new roles for NCC in the regulation of sodium, potassium, and blood pressure have been unraveled recently. For example, the recent discoveries that NCC is activated by angiotensin II but inhibited by dietary potassium shed light on how the kidney handles sodium during hypovolemia (high angiotensin II) and hyperkalemia. The additive effect of angiotensin II and aldosterone maximizes sodium reabsorption during hypovolemia, whereas the inhibitory effect of potassium on NCC increases delivery of sodium to the potassium-secreting portion of the nephron. In addition, great steps have been made in unraveling the molecular machinery that controls NCC. This complex network consists of kinases and ubiquitinases, including WNKs, SGK1, SPAK, Nedd4-2, Cullin-3, and Kelch-like 3. The pathophysiological significance of this network is illustrated by the fact that modification of each individual protein in the network changes NCC activity and results in salt-dependent hypotension or hypertension. This review aims to summarize these new insights in an integrated manner while identifying unanswered questions.




Similar content being viewed by others
References
Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH (2001) Loop diuretic infusion increases thiazide-sensitive Na(+)/Cl(−)-cotransporter abundance: role of aldosterone. J Am Soc Nephrol 12:1335–1341
Arroyo JP, Lagnaz D, Ronzaud C, Vazquez N, Ko BS, Moddes L, Ruffieux-Daidie D, Hausel P, Koesters R, Yang B, Stokes JB, Hoover RS, Gamba G, Staub O (2011) Nedd4-2 modulates renal Na+–Cl− cotransporter via the aldosterone-SGK1-Nedd4-2 pathway. J Am Soc Nephrol 22:1707–1719
Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G (2011) Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology (Bethesda) 26:115–123
Bazzini C, Vezzoli V, Sironi C, Dossena S, Ravasio A, De Biasi S, Garavaglia M, Rodighiero S, Meyer G, Fascio U, Furst J, Ritter M, Botta G, Paulmichl M (2005) Thiazide-sensitive NaCl-cotransporter in the intestine: possible role of hydrochlorothiazide in the intestinal Ca2+ uptake. J Biol Chem 280:19902–19910
Bindels RJ (2010) 2009 Homer W. Smith Award: minerals in motion: from new ion transporters to new concepts. J Am Soc Nephrol 21:1263–1269
Bostanjoglo M, Reeves WB, Reilly RF, Velazquez H, Robertson N, Litwack G, Morsing P, Dorup J, Bachmann S, Ellison DH (1998) 11beta-Hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na–Cl cotransporter expression by distal tubules. J Am Soc Nephrol 9:1347–1358
Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP (2012) Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482:98–102
Brater DC (1998) Diuretic therapy. N Engl J Med 339:387–395
Calo LA, Davis PA (2010) Number and function of circulating endothelial progenitor cells and calcitonin gene-related peptide in hypertension: support from and opportunities in Bartter’s and Gitelman’s syndromes patients. J Hypertens 28:2169–2170, author reply 2171
Calo L, Davis PA, Semplicini A (2002) Reduced content of alpha subunit of Gq protein content in monocytes of Bartter and Gitelman syndromes: relationship with vascular hyporeactivity. Kidney Int 61:353–354
Calo LA, Puato M, Schiavo S, Zanardo M, Tirrito C, Pagnin E, Balbi G, Davis PA, Palatini P, Pauletto P (2008) Absence of vascular remodelling in a high angiotensin-II state (Bartter’s and Gitelman’s syndromes): implications for angiotensin II signalling pathways. Nephrol Dial Transplant 23:2804–2809
Castaneda-Bueno M, Vazquez N, Bustos-Jaimes I, Hernandez D, Rodriguez-Lobato E, Pacheco-Alvarez D, Carino-Cortes R, Moreno E, Bobadilla NA, Gamba G (2010) A single residue in transmembrane domain 11 defines the different affinity for thiazides between the mammalian and flounder NaCl transporters. Am J Physiol Renal Physiol 299:F1111–F1119
Chavez-Canales M, Arroyo JP, Ko B, Vazquez N, Bautista R, Castaneda-Bueno M, Bobadilla NA, Hoover RS, Gamba G (2013) Insulin increases the functional activity of the renal NaCl cotransporter. J Hypertens 31:303–311
Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S (2008) Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74:1403–1409
Colussi G, Bettinelli A, Tedeschi S, De Ferrari ME, Syren ML, Borsa N, Mattiello C, Casari G, Bianchetti MG (2007) A thiazide test for the diagnosis of renal tubular hypokalemic disorders. Clin J Am Soc Nephrol 2:454–460
Costanzo LS (1985) Localization of diuretic action in microperfused rat distal tubules: Ca and Na transport. Am J Physiol 248:F527–F535
Cruz DN (2001) The renal tubular Na–Cl co-transporter (NCCT): a potential genetic link between blood pressure and bone density? Nephrol Dial Transplant 16:691–694
de Jong JC, Willems PH, Mooren FJ, van den Heuvel LP, Knoers NV, Bindels RJ (2003) The structural unit of the thiazide-sensitive NaCl cotransporter is a homodimer. J Biol Chem 278:24302–24307
Dimke H (2011) Exploring the intricate regulatory network controlling the thiazide-sensitive NaCl cotransporter (NCC). Pflugers Arch 462:767–777
Dvorak MM, De Joussineau C, Carter DH, Pisitkun T, Knepper MA, Gamba G, Kemp PJ, Riccardi D (2007) Thiazide diuretics directly induce osteoblast differentiation and mineralized nodule formation by interacting with a sodium chloride co-transporter in bone. J Am Soc Nephrol 18:2509–2516
Ellison DH (2012) Adaptation in Gitelman syndrome: “we just want to pump you up”. Clin J Am Soc Nephrol 7:379–382
Ellison DH, Loffing J (2009) Thiazide effects and adverse effects: insights from molecular genetics. Hypertension 54:196–202
Ellison DH, Velazquez H, Wright FS (1987) Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol 253:F546–F554
Ernst ME, Moser M (2009) Use of diuretics in patients with hypertension. N Engl J Med 361:2153–2164
Fanestil DD, Hyde RH, Blakely P, Vaughn DA (1999) Dietary magnesium, not calcium, regulates renal thiazide receptor. J Am Soc Nephrol 10:458–463
Faroqui S, Sheriff S, Amlal H (2006) Metabolic acidosis has dual effects on sodium handling by rat kidney. Am J Physiol Renal Physiol 291:F322–F331
Frindt G, Houde V, Palmer LG (2011) Conservation of Na+ vs. K+ by the rat cortical collecting duct. Am J Physiol Renal Physiol 301:F14–F20
Frindt G, Palmer LG (2009) Surface expression of sodium channels and transporters in rat kidney: effects of dietary sodium. Am J Physiol Renal Physiol 297:F1249–F1255
Gamba G (2009) The thiazide-sensitive Na+–Cl− cotransporter: molecular biology, functional properties, and regulation by WNKs. Am J Physiol Renal Physiol 297:F838–F848
Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC (1994) Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium–(potassium)–chloride cotransporter family expressed in kidney. J Biol Chem 269:17713–17722
Gamba G, Saltzberg SN, Lombardi M, Miyanoshita A, Lytton J, Hediger MA, Brenner BM, Hebert SC (1993) Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium–chloride cotransporter. Proc Natl Acad Sci U S A 90:2749–2753
Glaudemans B, Yntema HG, San-Cristobal P, Schoots J, Pfundt R, Kamsteeg EJ, Bindels RJ, Knoers NV, Hoenderop JG, Hoefsloot LH (2012) Novel NCC mutants and functional analysis in a new cohort of patients with Gitelman syndrome. Eur J Hum Genet 20:263–270
Glover M, Mercier Zuber A, Figg N, O’Shaughnessy KM (2010) The activity of the thiazide-sensitive Na(+)–Cl(−) cotransporter is regulated by protein phosphatase PP4. Can J Physiol Pharmacol 88:986–995
Glover M, Zuber AM, O’Shaughnessy KM (2009) Renal and brain isoforms of WNK3 have opposite effects on NCCT expression. J Am Soc Nephrol 20:1314–1322
Golbang AP, Cope G, Hamad A, Murthy M, Liu CH, Cuthbert AW, O’Shaughnessy KM (2006) Regulation of the expression of the Na/Cl cotransporter by WNK4 and WNK1: evidence that accelerated dynamin-dependent endocytosis is not involved. Am J Physiol Renal Physiol 291:F1369–F1376
Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA (2012) SPAK isoforms and OSR1 regulate sodium–chloride co-transporters in a nephron-specific manner. J Biol Chem 287:37673–37690
Hadchouel J, Delaloy C, Faure S, Achard JM, Jeunemaitre X (2006) Familial hyperkalemic hypertension. J Am Soc Nephrol 17:208–217
Hadchouel J, Soukaseum C, Busst C, Zhou XO, Baudrie V, Zurrer T, Cambillau M, Elghozi JL, Lifton RP, Loffing J, Jeunemaitre X (2010) Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension. Proc Natl Acad Sci U S A 107:18109–18114
Hasegawa M, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y (2007) Multidrug resistance-associated protein 4 is involved in the urinary excretion of hydrochlorothiazide and furosemide. J Am Soc Nephrol 18:37–45
Hebert SC, Mount DB, Gamba G (2004) Molecular physiology of cation-coupled Cl− cotransport: the SLC12 family. Pflugers Arch 447:580–593
Hoorn EJ, Ellison DH (2012) WNK kinases and the kidney. Exp Cell Res 318:1020–1026
Hoorn EJ, Nelson JH, McCormick JA, Ellison DH (2011) The WNK kinase network regulating sodium, potassium, and blood pressure. J Am Soc Nephrol 22:605–614
Hoorn EJ, Pisitkun T, Zietse R, Gross P, Frokiaer J, Wang NS, Gonzales PA, Star RA, Knepper MA (2005) Prospects for urinary proteomics: exosomes as a source of urinary biomarkers. Nephrol (Carlton) 10:283–290
Hoorn EJ, Walsh SB, McCormick JA, Furstenberg A, Yang CL, Roeschel T, Paliege A, Howie AJ, Conley J, Bachmann S, Unwin RJ, Ellison DH (2011) The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 17:1304–1309
Hoorn EJ, Walsh SB, McCormick JA, Zietse R, Unwin RJ, Ellison DH (2012) Pathogenesis of calcineurin inhibitor-induced hypertension. J Nephrol 25:269–275
Hoorn EJ, Walsh SB, Unwin RJ, Ellison DH (2012) Hypertension after kidney transplantation: calcineurin inhibitors increase salt-sensitivity. J Hypertens 30:832–833, author reply 833–834
Hoorn EJ, Zietse R (2008) Hyponatremia revisited: translating physiology to practice. Nephron Physiol 108:p46–p59
Hoorn EJ, Zietse R (2013) Disorders of calcium and magnesium balance: a physiology-based approach. Pediatr Nephrol 28:1195–1206
Hoover RS (2011) Angiotensin II: a candidate for an aldosterone-independent mediator of potassium preservation during volume depletion. Kidney Int 79:377–379
Hoover RS, Poch E, Monroy A, Vazquez N, Nishio T, Gamba G, Hebert SC (2003) N-Glycosylation at two sites critically alters thiazide binding and activity of the rat thiazide-sensitive Na(+):Cl(−) cotransporter. J Am Soc Nephrol 14:271–282
Hossain Khan MZ, Sohara E, Ohta A, Chiga M, Inoue Y, Isobe K, Wakabayashi M, Oi K, Rai T, Sasaki S, Uchida S (2012) Phosphorylation of Na–Cl cotransporter by OSR1 and SPAK kinases regulates its ubiquitination. Biochem Biophys Res Commun 425:456–461
Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP (2008) Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 40:592–599
Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA (1998) The thiazide-sensitive Na–Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A 95:14552–14557
Ko B, Joshi LM, Cooke LL, Vazquez N, Musch MW, Hebert SC, Gamba G, Hoover RS (2007) Phorbol ester stimulation of RasGRP1 regulates the sodium–chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci U S A 104:20120–20125
Ko B, Kamsteeg EJ, Cooke LL, Moddes LN, Deen PM, Hoover RS (2010) RasGRP1 stimulation enhances ubiquitination and endocytosis of the sodium–chloride cotransporter. Am J Physiol Renal Physiol 299:F300–F309
Ko B, Mistry AC, Hanson LN, Mallick R, Wynne BM, Thai TL, Bailey JL, Klein JD, Hoover RS (2013) Aldosterone acutely stimulates NCC activity via a SPAK-mediated pathway. Am J Physiol Renal Physiol 305:F645–F652
Komers R, Rogers S, Oyama TT, Xu B, Yang CL, McCormick J, Ellison DH (2012) Enhanced phosphorylation of Na(+)–Cl− co-transporter in experimental metabolic syndrome: role of insulin. Clin Sci (Lond) 123:635–647
Konrad M, Vollmer M, Lemmink HH, van den Heuvel LP, Jeck N, Vargas-Poussou R, Lakings A, Ruf R, Deschenes G, Antignac C, Guay-Woodford L, Knoers NV, Seyberth HW, Feldmann D, Hildebrandt F (2000) Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J Am Soc Nephrol 11:1449–1459
Kriz W, Bankir L (1988) A standard nomenclature for structures of the kidney. The Renal Commission of the International Union of Physiological Sciences (IUPS). Kidney Int 33:1–7
Kunau RT Jr, Weller DR, Webb HL (1975) Clarification of the site of action of chlorothiazide in the rat nephron. J Clin Invest 56:401–407
Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP (2006) Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38:1124–1132
Ledeganck KJ, Boulet GA, Horvath CA, Vinckx M, Bogers JJ, Van Den Bossche R, Verpooten GA, De Winter BY (2011) Expression of renal distal tubule transporters TRPM6 and NCC in a rat model of cyclosporine nephrotoxicity and effect of EGF treatment. Am J Physiol Renal Physiol 301:F486–F493
Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D (2010) The Na+-dependent chloride–bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120:1627–1635
Lin SH, Cheng NL, Hsu YJ, Halperin ML (2004) Intrafamilial phenotype variability in patients with Gitelman syndrome having the same mutations in their thiazide-sensitive sodium/chloride cotransporter. Am J Kidney Dis 43:304–312
Lin SH, Shiang JC, Huang CC, Yang SS, Hsu YJ, Cheng CJ (2005) Phenotype and genotype analysis in Chinese patients with Gitelman’s syndrome. J Clin Endocrinol Metab 90:2500–2507
Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, Bloch-Faure M, Hoenderop JG, Shull GE, Meneton P, Kaissling B (2004) Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol 15:2276–2288
Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F (2001) Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol 280:F675–F682
Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, International Consortium for Blood P, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X (2012) KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44:456–460, S451-453
Masilamani S, Wang X, Kim GH, Brooks H, Nielsen J, Nielsen S, Nakamura K, Stokes JB, Knepper MA (2002) Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC, and ENaC abundance changes with dietary NaCl restriction. Am J Physiol Renal Physiol 283:F648–F657
Mastroianni N, De Fusco M, Zollo M, Arrigo G, Zuffardi O, Bettinelli A, Ballabio A, Casari G (1996) Molecular cloning, expression pattern, and chromosomal localization of the human Na–Cl thiazide-sensitive cotransporter (SLC12A3). Genomics 35:486–493
Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z (2002) Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab 87:3248–3254
McCormick JA, Ellison DH (2011) The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev 91:177–219
McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH (2011) A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14:352–364
McCormick JA, Nelson JH, Yang CL, Curry JN, Ellison DH (2011) Overexpression of the sodium chloride cotransporter is not sufficient to cause familial hyperkalemic hypertension. Hypertension 58:888–894
McDonough AA (2010) Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol 298:R851–R861
Melnikov S, Mayan H, Uchida S, Holtzman EJ, Farfel Z (2011) Cyclosporine metabolic side effects: association with the WNK4 system. Eur J Clin Invest 41:1113–1120
Meneton P, Loffing J, Warnock DG (2004) Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol 287:F593–F601
Moreno E, Cristobal PS, Rivera M, Vazquez N, Bobadilla NA, Gamba G (2006) Affinity-defining domains in the Na–Cl cotransporter: a different location for Cl− and thiazide binding. J Biol Chem 281:17266–17275
Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H (2005) WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280:42685–42693
Morris RG, Hoorn EJ, Knepper MA (2006) Hypokalemia in a mouse model of Gitelman’s syndrome. Am J Physiol Renal Physiol 290:F1416–F1420
Moser M, Feig PU (2009) Fifty years of thiazide diuretic therapy for hypertension. Arch Intern Med 169:1851–1856
Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T (2011) Epigenetic modulation of the renal beta-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med 17:573–580
Mutig K, Saritas T, Uchida S, Kahl T, Borowski T, Paliege A, Bohlick A, Bleich M, Shan Q, Bachmann S (2010) Short-term stimulation of the thiazide-sensitive Na+–Cl− cotransporter by vasopressin involves phosphorylation and membrane translocation. Am J Physiol Renal Physiol 298:F502–F509
Nguyen MT, Lee DH, Delpire E, McDonough AA (2013) Differential regulation of Na+ transporters along nephron during AngII-dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol 305:F510–F519
Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ (2005) Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 115:1651–1658
Novello FC, Sprague JM (1957) Benzothiadiazine dioxides as novel diuretics. J Am Chem Soc 79:2028–2029
Ohta A, Rai T, Yui N, Chiga M, Yang SS, Lin SH, Sohara E, Sasaki S, Uchida S (2009) Targeted disruption of the Wnk4 gene decreases phosphorylation of Na–Cl cotransporter, increases Na excretion and lowers blood pressure. Hum Mol Genet 18:3978–3986
Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T (2013) The CUL3-KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J 451:111–122
O’Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW (2006) Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J Am Soc Nephrol 17:2402–2413
Pacheco-Alvarez D, Vazquez N, Castaneda-Bueno M, de-Los-Heros P, Cortes-Gonzalez C, Moreno E, Meade P, Bobadilla NA, Gamba G (2012) WNK3-SPAK interaction is required for the modulation of NCC and other members of the SLC12 family. Cell Physiol Biochem 29:291–302
Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA (2010) Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78:160–169
Pickkers P, Hughes AD, Russel FG, Thien T, Smits P (1998) Thiazide-induced vasodilation in humans is mediated by potassium channel activation. Hypertension 32:1071–1076
Pisitkun T, Shen RF, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101:13368–13373
Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O’Shaughnessy KM, Alessi DR (2010) Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med 2:63–75
Renfro JL (1975) Water and ion transport by the urinary bladder of the teleost Pseudopleuronectes americanus. Am J Physiol 228:52–61
Renfro JL (1977) Interdependence of Active Na+ and Cl− transport by the isolated urinary bladder of the teleost, Pseudopleuronectes americanus. J Exp Zool 199:383–390
Rinehart J, Kahle KT, de Los Heros P, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP (2005) WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci U S A 102:16777–16782
Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP (2007) An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci U S A 104:4025–4029
Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, Boase NA, Perrier R, Maillard M, Yang B, Stokes JB, Koesters R, Kumar S, Hummler E, Loffing J, Staub O (2013) Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest 123:657–665
Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yang YF, David LL, Zhu X, Yang CL, Ellison DH (2009) Aldosterone mediates activation of the thiazide-sensitive Na–Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest 119:2601–2612
San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G (2009) Angiotensin II signaling increases activity of the renal Na–Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci U S A 106:4384–4389
San-Cristobal P, Ponce-Coria J, Vazquez N, Bobadilla NA, Gamba G (2008) WNK3 and WNK4 amino-terminal domain defines their effect on the renal Na+–Cl− cotransporter. Am J Physiol Renal Physiol 295:F1199–F1206
Sandberg MB, Maunsbach AB, McDonough AA (2006) Redistribution of distal tubule Na+–Cl− cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol 291:F503–F508
Saritas T, Borschewski A, McCormick JA, Paliege A, Dathe C, Uchida S, Terker A, Himmerkus N, Bleich M, Demaretz S, Laghmani K, Delpire E, Ellison DH, Bachmann S, Mutig K (2013) SPAK differentially mediates vasopressin effects on sodium cotransporters. J Am Soc Nephrol 24:407–418
Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE (1998) Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+–Cl− cotransporter of the distal convoluted tubule. J Biol Chem 273:29150–29155
Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP (2013) Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci U S A 110:7838–7843
Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP (1996) Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na–Cl cotransporter. Nat Genet 12:24–30
Sohara E, Rai T, Yang SS, Ohta A, Naito S, Chiga M, Nomura N, Lin SH, Vandewalle A, Ohta E, Sasaki S, Uchida S (2011) Acute insulin stimulation induces phosphorylation of the Na–Cl cotransporter in cultured distal mpkDCT cells and mouse kidney. PLoS One 6:e24277
Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA (2006) Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol 290:F1055–F1064
Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J (2013) Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83:811–824
Stokes JB (1984) Sodium chloride absorption by the urinary bladder of the winter flounder. A thiazide-sensitive, electrically neutral transport system. J Clin Invest 74:7–16
Subramanya AR, Yang CL, McCormick JA, Ellison DH (2006) WNK kinases regulate sodium chloride and potassium transport by the aldosterone-sensitive distal nephron. Kidney Int 70:630–634
Tseng MH, Yang SS, Hsu YJ, Fang YW, Wu CJ, Tsai JD, Hwang DY, Lin SH (2012) Genotype, phenotype, and follow-up in Taiwanese patients with salt-losing tubulopathy associated with SLC12A3 mutation. J Clin Endocrinol Metab 97:E1478–E1482
Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, Nigam SK (2008) Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol 294:F867–F873
Vallon V, Schroth J, Lang F, Kuhl D, Uchida S (2009) Expression and phosphorylation of the Na+–Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297:F704–F712
van Angelen AA, Glaudemans B, van der Kemp AW, Hoenderop JG, Bindels RJ (2013) Cisplatin-induced injury of the renal distal convoluted tubule is associated with hypomagnesaemia in mice. Nephrol Dial Transplant 28:879–889
van der Lubbe N, Jansen PM, Salih M, Fenton RA, van den Meiracker AH, Danser AH, Zietse R, Hoorn EJ (2012) The phosphorylated sodium chloride cotransporter in urinary exosomes is superior to prostasin as a marker for aldosteronism. Hypertension 60:741–748
van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ (2011) Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int 79:66–76
van der Lubbe N, Lim CH, Meima ME, van Veghel R, Rosenbaek LL, Mutig K, Danser AH, Fenton RA, Zietse R, Hoorn EJ (2012) Aldosterone does not require angiotensin II to activate NCC through a WNK4-SPAK-dependent pathway. Pflugers Arch 463:853–863
Van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ (2013) K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl- cotransporter. Am J Physiol Renal Physiol 305(8):F1177–88
van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ (2013) K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+–Cl− cotransporter. Am J Physiol Renal Physiol 305:F1177–F1188
van der Lubbe N, Zietse R, Hoorn EJ (2013) Effects of angiotensin II on kinase-mediated sodium and potassium transport in the distal nephron. Curr Opin Nephrol Hypertens 22:120–126
Vargas-Poussou R, Dahan K, Kahila D, Venisse A, Riveira-Munoz E, Debaix H, Grisart B, Bridoux F, Unwin R, Moulin B, Haymann JP, Vantyghem MC, Rigothier C, Dussol B, Godin M, Nivet H, Dubourg L, Tack I, Gimenez-Roqueplo AP, Houillier P, Blanchard A, Devuyst O, Jeunemaitre X (2011) Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol 22:693–703
Velazquez H, Bartiss A, Bernstein P, Ellison DH (1996) Adrenal steroids stimulate thiazide-sensitive NaCl transport by rat renal distal tubules. Am J Physiol 270:F211–F219
Verlander JW, Tran TM, Zhang L, Kaplan MR, Hebert SC (1998) Estradiol enhances thiazide-sensitive NaCl cotransporter density in the apical plasma membrane of the distal convoluted tubule in ovariectomized rats. J Clin Invest 101:1661–1669
Wagner CA, Loffing-Cueni D, Yan Q, Schulz N, Fakitsas P, Carrel M, Wang T, Verrey F, Geibel JP, Giebisch G, Hebert SC, Loffing J (2008) Mouse model of type II Bartter’s syndrome. II. Altered expression of renal sodium- and water-transporting proteins. Am J Physiol Renal Physiol 294:F1373–F1380
Wagner CA, Ott M, Klingel K, Beck S, Melzig J, Friedrich B, Wild KN, Broer S, Moschen I, Albers A, Waldegger S, Tummler B, Egan ME, Geibel JP, Kandolf R, Lang F (2001) Effects of the serine/threonine kinase SGK1 on the epithelial Na(+) channel (ENaC) and CFTR: implications for cystic fibrosis. Cell Physiol Biochem 11:209–218
Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S (2013) Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep 3:858–868
Wei Y, Zavilowitz B, Satlin LM, Wang WH (2007) Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem 282:6455–6462
Welling PA, Chang YP, Delpire E, Wade JB (2010) Multigene kinase network, kidney transport, and salt in essential hypertension. Kidney Int 77:1063–1069
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP (2001) Human hypertension caused by mutations in WNK kinases. Science 293:1107–1112
Wu G, Peng JB (2013) Disease-causing mutations in KLHL3 impair its effect on WNK4 degradation. FEBS Lett 587:1717–1722
Xu JZ, Hall AE, Peterson LN, Bienkowski MJ, Eessalu TE, Hebert SC (1997) Localization of the ROMK protein on apical membranes of rat kidney nephron segments. Am J Physiol 273:F739–F748
Yang CL, Angell J, Mitchell R, Ellison DH (2003) WNK kinases regulate thiazide-sensitive Na–Cl cotransport. J Clin Invest 111:1039–1045
Yang SS, Fang YW, Tseng MH, Chu PY, Yu IS, Wu HC, Lin SW, Chau T, Uchida S, Sasaki S, Lin YF, Sytwu HK, Lin SH (2013) Phosphorylation regulates NCC stability and transporter activity in vivo. J Am Soc Nephrol 24:1587–1597
Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH (2010) SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21:1868–1877
Yang LE, Sandberg MB, Can AD, Pihakaski-Maunsbach K, McDonough AA (2008) Effects of dietary salt on renal Na+ transporter subcellular distribution, abundance, and phosphorylation status. Am J Physiol Renal Physiol 295:F1003–F1016
Yang CL, Zhu X, Ellison DH (2007) The thiazide-sensitive Na–Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest 117:3403–3411
Yang CL, Zhu X, Wang Z, Subramanya AR, Ellison DH (2005) Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J Clin Invest 115:1379–1387
Yue P, Sun P, Lin DH, Pan C, Xing W, Wang W (2011) Angiotensin II diminishes the effect of SGK1 on the WNK4-mediated inhibition of ROMK1 channels. Kidney Int 79:423–431
Zhou B, Wang D, Feng X, Zhang Y, Wang Y, Zhuang J, Zhang X, Chen G, Delpire E, Gu D, Cai H (2012) WNK4 inhibits NCC protein expression through MAPK ERK1/2 signaling pathway. Am J Physiol Renal Physiol 302:F533–F539
Zhou B, Zhuang J, Gu D, Wang H, Cebotaru L, Guggino WB, Cai H (2010) WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J Am Soc Nephrol 21:82–92
Acknowledgments
EJH is supported by the Dutch Kidney Foundation (KJPB 08.004) and the Netherlands Organisation for Scientific Research (Veni). NvdL is supported by the Netherlands Foundation for Cardiovascular Excellence. JL is supported by the Swiss National Science Foundation, the Swiss National Centre in Competence in Research Kidney CH, and the Zurich Centre for Integrative Human Physiology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moes, A.D., van der Lubbe, N., Zietse, R. et al. The sodium chloride cotransporter SLC12A3: new roles in sodium, potassium, and blood pressure regulation. Pflugers Arch - Eur J Physiol 466, 107–118 (2014). https://doi.org/10.1007/s00424-013-1407-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1407-9