Abstract
Main conclusion
The ratio of nicotianamine to deoxymugenic acid controls tissue-specific metal homeostasis in rice and regulates metal delivery to the endosperm.
Abstract
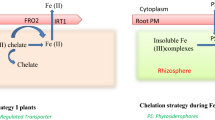
The metal-chelating phytosiderophores nicotianamine (NA) and 2′deoxymugenic acid (DMA) are significant factors for the control of metal homeostasis in graminaceous plants. These compounds are thought to influence metal homeostasis, but their individual roles and the effect of altering the NA:DMA ratio are unknown. We purposely generated rice lines with high and low NA:DMA ratios (HND and LND lines, respectively). The HND lines accumulated more iron (Fe), zinc (Zn), manganese (Mn) and copper (Cu) in the endosperm through the mobilization of Fe, Zn and Mn from the seed husk to the endosperm. In contrast, Fe, Zn and Mn were mobilized to the husk in the LND lines, whereas Cu accumulated in the endosperm. Different groups of metals are, therefore, taken up, transported and sequestered in vegetative tissues in the HND and LND lines to achieve this metal distribution pattern in the seeds. We found that different sets of endogenous metal homeostasis genes were modulated in the HND and LND lines to achieve differences in metal homeostasis. Our findings demonstrate that the NA:DMA ratio is a key factor regulating metal homeostasis in graminaceous plants. These findings can help formulate refined strategies to improve nutrient composition and nutrient use efficiency in crop plants.






Similar content being viewed by others
References
Ayoma T, Kobayashi T, Takahashi M et al (2009) OsYSL18 is a rice iron (III)-deoxymugenic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol Biol 70:681–692
Banakar R, Alvarez-Fernandez A, Abadia J et al (2017a) The expression of heterologous Fe(III) phytosiderophore transporter HvYS1 in rice increases Fe uptake, translocation and seed loading and excludes heavy metals by selective Fe transport. Plant Biotechnol J 15:423–432
Banakar R, Alvarez-Fernández Á, Díaz-Benito P et al (2017b) Phytosiderophores determine thresholds for iron and zinc accumulation in biofortified rice endosperm while inhibiting the accumulation of cadmium. J Exp Bot 17:4983–4995
Bashir K, Takahashi R, Akhtar S et al (2013) The knockdown of OsVIT2 and MIT affects iron localization in rice seed. Rice (N Y) 6:31
Benes I, Schreiber K, Ripperger H et al (1983) Metal complex formation of nicotianamine, a possible phytosiderophore. Experientia 39:261–262
Cheng L, Wang F, Shou H et al (2007) Mutation in nicotianamine aminotransferase stimulated the Fe(II) acquisition system and led to iron accumulation in rice. Plant Physiol 145:1647–1657
Christensen AH, Quail PH (1996) Ubiquitin promoter based vectors for high-level expression of selectable and/or screenable marker genes in mono-cotyledonous plants. Trans Res 5:213–218
Christou P, Ford TL (1995) The impact of selection parameters on the phenotype and genotype of transgenic rice callus and plants. Trans Res 4:44–51
Christou P, Ford TL, Kofron M (1991) Production of transgenic rice (Oryza Sativa L.) plants from agronomically important indica and japonica varieties via electric discharge particle acceleration of exogenous DNA into immature zygotic embryos. Bio/Technology 9:957–962
Clemens S, Aarts MGM, Thomine S et al (2013) Plant Science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18:1–8
Curie C, Cassin G, Couch D et al (2009) Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 103:1–11
Díaz-Benito P, Banakar R, Rodríguez-Menéndez SM, Capell T, Pereiro R, Christou P, Abadía J, Fernández B, Álvarez-Fernández A (2018) Distribution of iron and zinc between the embryo and endosperm of rice (Oryza sativa L.) seeds in contrasting nicotianamine/2′-deoxymugineic acid scenarios. Front Plant Sci 9:1190
Inoue H, Higuchi K, Takahashi M et al (2003) Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J 36:366–381
Ishimaru Y, Suzuki M, Kobayashi T et al (2005) OsZIP4, a novel zinc-regulated zinc transporter in rice. J Exp Bot 56:3207–3214
Ishimaru Y, Masuda H, Bashir K et al (2010) Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J 62:379–390
Jiang W, Struik PC, Lingna J et al (2007) Uptake and distribution of root applied and foliar applied 65Zn after flowering in aerobic rice. Ann App Biol 150:383–391
Johnson AAT, Kyriacou B, Callahan DL et al (2011) Constitutive overexpression of the OsNAS gene family reveals single-gene strategies for effective iron- and zinc-biofortification of rice endosperm. PLoS ONE 6:e24476
Kakei Y, Ishimaru Y, Kobayashi T et al (2012) OsYSL16 plays a role in the allocation of iron. Plant Mol Biol 79:583–594
Kobayashi T, Suzuki M, Inoue H et al (2005) Expression of iron-acquisition-related genes in iron-deficient rice is coordinately induced by partially conserved iron-deficiency responsive elements. J Exp Bot 56:1305–1316
Kobayashi T, Itai RN, Ogo Y et al (2009) The rice transcription factor IDEF1 is essential for the early response to iron deficiency, and induces vegetative expression of late embryogenesis abundant genes. Plant J 60:948–961
Lee S, An G (2009) Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ 32:408–416
Lee S, Jeon US, Lee SJ et al (2009) Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proc Nat Acad Sci USA 106:22014–22019
Lee S, Kim Y, Jeon U et al (2012a) Activation of rice nicotianamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Mol Cells 33:269–275
Lee S, Ryoo N, Jeon JS et al (2012b) Activation of rice yellow stripe1-like 16 (OsYSL16) enhances iron efficiency. Mol Cells 33:117–126
Ma JF, Taketa S, Chang YC et al (1999) Biosynthesis of phytosiderophores in several Triticeae species with different genomes. J Exp Bot 50:723–726
Masuda H, Usuda K, Kobayashi T et al (2009) Overexpression of the barley nicotianamine synthase gene HvNAS1 increases iron and zinc concentrations in rice grains. Rice 2:155–166
Masuda H, Ishimaru Y, Aung MS et al (2012) Iron biofortification in rice by the introduction of multiple genes involved in iron nutrition. Sci Rep 2:543
Mino Y, Ishida T, Ota N et al (1981) Molecular structure and spectroscopic properties of copper (II) complex with mugenic acid, a novel amino acid from graminaceous plants. Inorg Chem 20:3440–3444
Moreno-Moyano LT, Bonneau JP, Sánchez-Palacios JT, Tohme J, Johnson AAT (2016) Association of increased grain iron and zinc concentrations with agro-morphological traits of biofortified rice. Front Plant Sci 7:1463
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ chem Lett 8:199–216
Nishiyama R, Kato M, Nagata S et al (2012) Identification of Zn-nicotianamine and Fe-2-deoxymugineic acid in the phloem sap from rice plants (Oryza sativa L). Plant Cell Physiol 53:381–390
Ramegowda Y, Venkategowda R, Jagadish P et al (2013) Expression of a rice Zn transporter, OsZIP1 increases Zn concentration in tobacco and fingermillet transgenic plants. Plant Biotechnol Rep 7:309–319
Ramesh SA, Shin R, Eide DJ et al (2003) Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol 133:126–134
Sasaki A, Yamaji N, Yokosho K, Ma JF (2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24:2155–2167
Sasaki A, Yamaji N, Ma JF (2014) Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J Exp Bot 65:6013–6021
Sperotto RA (2013) Zn/Fe remobilization from vegetative tissues to rice seeds: should I stay or should I go? Ask Zn/Fe supply. Front Plant Sci 4:1–4
Sperotto RA, Ricachenevsky FK, WaldowVde A et al (2012) Iron biofortification in rice: it’s a long way to the top. Plant Sci 190:24–39
Sperotto RA, Ricachenevsky FK, Waldow VdeA, Muller ALH, Dressler VL, Fett JP (2013) Rice grain Fe, Mn and Zn accumulation: How important are flag leaves and seed number? Plant Soil Environ 59:262–266
Sudhakar D, Duc LT, Bong BB et al (1998) An efficient rice transformation system utilizing mature seed-derived explants and a portable, inexpensive particle bombardment device. Trans Res 7:289–294
Suzuki M, Tsukamoto T, Inoue H et al (2008) Deoxymugineic acid increases Zn translocation in Zn deficient rice plant. Plant Mol Biol 66:609–617
Takahashi M, Nakanishi H, Kawasaki S et al (2001) Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat Biotechnol 19:466–469
Takahashi R, Ishimaru Y, Senoura T, Shimo H, Ishikawa S, Arao T, Nakanishi H, Nishizawa NK (2011) The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J Exp Bot 62:4843–4850
Takahashi R, Ishimaru Y, Shimo H et al (2012) The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ 35:1948–1957
Trijatmiko KR, Dueñas C, Tsakirpaloglou N et al (2016) Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci Rep 6:19792–19805
Valdez M, Cabrera-Ponce JL, Sudhakhar D et al (1998) Transgenic Central American, West African and Asian elite rice varieties resulting from particle bombardment of foreign DNA into mature seed-derived explants utilizing three different bombardment devices. Ann Bot 82:795–801
Vert G, Grotz N, Dedaldechamp F et al (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233
Vigani G, Zocchi G, Bashir K et al (2013) Cellular iron homeostasis and metabolism in plants. Front Plant Sci 4:1–3
Wang M, Gruissem W, Bhullar NK (2013) Nicotianamine synthase overexpression positively modulates iron homeostasis-related genes in high iron rice. Front Plant Sci 4:156
Yamaji N, Xia J, Mitani-Ueno N et al (2013) Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol 162:927–939
Yang M, Zhang Y, Zhang L et al (2014) OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J Exp Bot 65:4849–4861
Yokosho K, Yamaji N, Ueno D et al (2009) OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol 149:297–305
Yoneyama T, Gosho T, Kato M et al (2010) Xylem and phloem transport of Cd, Zn and Fe into the grains of rice plants (Oryza sativa L.) grown continuously flooded Cd contaminated soil. Soil Sci Plant Nutr 56:445–453
Yuan M, Li X, Xiao J et al (2011) Molecular and functional analysis of COPT/ctr-type copper transporter—like gene family in rice. BMC Plant Biol 11:69–78
Zhang Y, Xu HY, Yi YH et al (2012) Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J 72:400–410
Zheng L, Yamaji N, Yokosho K et al (2012) YSL16 is a phloem-localized transporter of the copper-nicotianamine complex that is responsible for copper distribution in Rice. Plant Cell 24:3767–3782
Acknowledgements
We acknowledge support from the European Research Council IDEAS Advanced Grant Program (BIOFORCE) to P.C., Generalitat de Catalunya Grant 2017 SGR 828 to ABBU (BIO2014-54426-P) and the Spanish Ministry of Economy and Competitivity (MINECO; projects AGL2016-75226-R, co-financed with FEDER) and the Aragón Government (Group A09_17R) to J.A. R.B was supported by a PhD fellowship from the University of Lleida, Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1.
Fold changes in relative expression levels of endogenous genes involved in metal homeostasis. Table S2. Mass spectrometer settings for the quantitation of NA and DMA levels in transgenic plants. Table S3. Genes and primers used for quantitative real-time RT-PCR analysis. Supplementary material 1 (DOC 103 kb)
Rights and permissions
About this article
Cite this article
Banakar, R., Fernandez, A.A., Zhu, C. et al. The ratio of phytosiderophores nicotianamine to deoxymugenic acid controls metal homeostasis in rice. Planta 250, 1339–1354 (2019). https://doi.org/10.1007/s00425-019-03230-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-019-03230-2