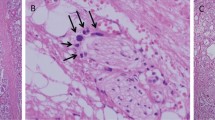
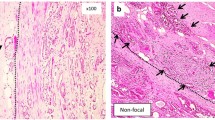
Abstract
Focal or non-focal/extensive extraprostatic extension of prostate carcinoma is an important pathologic prognostic parameter to be reported after radical prostatectomy. Currently, there is no agreement on how to measure and what are the best cutoff points to be used in practice. We hypothesized that digital microscopy would potentially provide more objective measurements of extraprostatic extension, thus better defining its clinical significance. To further our knowledge on digital prostate pathology, we evaluated the status of extraprostatic extension in 107 consecutive laparoscopic radical prostatectomy samples, using digital and conventional light microscopy. Mean linear and radial measurements of extraprostatic extension by digital microscopy significantly correlated to pT status (p = 0.022 and p = 0.050, respectively) but only radial measurements correlated to biochemical recurrence (p = 0.042) and grade groups (p = 0.022). None of the measurements, whether conventional or digital, were associated with lymph node status. Receiving operating characteristic analysis showed a potential cutoff point to assess linear measurements by conventional (< vs. > 24.21 mm) or digital microscopy (< vs. > 15 mm) or by radial measurement (< vs. > 1.6 mm). Finally, we observed an association between the number of paraffin blocks bearing EPE with pT (p = 0.041) status (digital microscopy), and linear measurements by conventional (p = 0.044) or digital microscopy (p = 0.045) with lymph node status. Reporting EPE measurements by digital microscopy, both linear and radial, and the number of paraffin blocks with EPE, might provide additional prognostic features after radical prostatectomy.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Volavšek M, Blanca A, Montironi R, Cheng L, Raspollini MR, Vau N, Fonseca J, Pierconti F, Lopez-Beltran A (2018) Digital versus light microscopy assessment of surgical margin status after radical prostatectomy. Virchows Arch 472:451–460. https://doi.org/10.1007/s00428-018-2296-2
Kench JG, Judge M, Delahunt B, Humphrey PA, Kristiansen G, Oxley J, Rasiah K, Takahashi H, Trpkov K, Varma M, Wheeler TM, Zhou M, Srigley JR, Egevad L (2019) Dataset for the reporting of prostate carcinoma in radical prostatectomy specimens: updated recommendations from the international collaboration on cancer reporting. Virchows Arch 475:263–277
Cheng L, Darson MF, Bergstralh EJ, Slezak J, Myers RP, Bostwick DG (1999) Correlation of margin status and extraprostatic extension with progression of prostate carcinoma. Cancer 86:1775–1782
Buyyounouski MK, Choyke PL, Kattan MW, McKenney JK, Srigley JR, Barocas DA, Brimo F, Brookland RK, Epstein JI, Fine SW, Halabi S, Hamstra DA, Mason MD, Oh WK, Pettaway CA, Sartor O, Schymura MJ, Touijer KA, Zelefsky MJ, Sandler HM, Amin MB, Lin DW (2017) Prostate. In: Amin MA (ed) AJCC cancer staging manual, American Joint Committee on Cancer, 8th edn. Springer, Chicago, pp 715–726
Chan SM, Garcia FJ, Moussa M, Gabril MY (2011) The clinical significance of in-depth pathological assessment of extraprostatic extension and margin status in radical prostatectomies for prostate cancer. Prostate Cancer Prostatic Dis 14:307–312
Davis BJ, Pisansky TM, Wilson TM, Rothenberg HJ, Pacelli A, Hillman DW, Sargent DJ, Bostwick DG (1999) The radial distance of extraprostatic extension of prostate carcinoma. Implications for prostate brachytheraphy. Cancer 85:2630–2637
Epstein JI, Carmichael MJ, Pizov G, Walsh PC (1993) Influence of capsular penetration on progression following radical prostatectomy: a study of 196 cases with long-term follow up. J Urol 150:135–141
Wheeler TM, Dillioglugil O, Kattan MW, Arakawa A, Soh S, Suyama K, Ohori M, Scardino PT (1998) Clinical and pathological significance of the level and extent of capsular invasion in clinical stage T1-2 prostate cancer. Hum Pathol 29:856–862
Jeong BC, Chalfin HJ, Lee SB, Feng Z, Epstein JI, Trock BJ, Partin AW, Humphreys E, Walsh PC, Han M (2015) The relationship between the extent of extraprostatic extension and survival following radical prostatectomy. Eur Urol 67:342–346
Magi-Galuzzi C, Evans AJ, Delahunt B, Epstein JI, Griffiths DF, van der Kwast TH, Montironi R, Wheeler TM, Srigley JR, Egevad L, Humphrey PA, and the ISUP Prostate Cancer Group (2011) International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 3: extraprostatic extension, lymphovascular invasion and locally advanced disease. Mod Pathol 24:26–38
Pan CC (2012) Significance of prostatic capsular status in radical prostatectomy. Urol Sci 23:15–17
Sohayda C, Kupelian PA, Levin HS, Klein EA (2000) Extent of extracapsular extension in localized prostate cancer. Urology 55:382–386
Sung MT, Lin HL, Koch MO, Davidson DD, Cheng L (2007) Radial distance of extraprostatic extension measured by ocular micrometer is an independent predictor of prostate-specific antigen recurrence. A new proposal for the substaging of pT3a prostate cancer. Am J Surg Pathol 31:311–318
Sung MT, Eble JN, Cheng L (2006) Invasion of fat justifies assignment of stage pT3a in prostatic adenocarcinoma. Pathology 38:309–311
Tan PH, Cheng L, Srigley JR, Griffiths D, Humphrey PA, van der Kwast TH, Montironi R, Wheeler TM, Delahunt B, Egevad L, Epstein JI, and the ISUP Prostate Cancer Group (2011) International Society of Urological Pathology (ISUP) Consenus conference on handling and staging of radical prostatectomy specimens. Working group 5: surgical margins. Mod Pathol 24:48–57
Mehralivand S, Shih JH, Harmon S, Smith C, Bloom J, Czarniecki M, Gold S, Hale G, Rayn K, Merino MJ, Wood BJ, Pinto PA, Choyke PL, Turkbey B (2019) A grading system for the assessment of risk of extraprostatic extension of prostate cancer at multiparametric MRI. Radiology 290:709–719. https://doi.org/10.1148/radiol.2018181278
Flood TA, Schieda N, Keefe DT, Breau RH, Morash C, Hogan K, Belanger EC, Mai KT, Robertson SJ (2016) Utility of Gleason pattern 4 morphologies detected on transrectal ultrasound (TRUS)-guided biopsies for prediction of upgrading or upstaging in Gleason score 3 + 4 = 7 prostate cancer. Virchows Arch 469:313–319. https://doi.org/10.1007/s00428-016-1981-2
Anderson BB, Oberlin DT, Razmaria AA, Choy B, Zagaja GP, Shalhav AL, Meeks JJ, Yang XJ, Paner GP, Eggener SE (2017) Extraprostatic extension is extremely rare for contemporary Gleason score 6 prostate cancer. Eur Urol 72:455–460. https://doi.org/10.1016/j.eururo.2016.11.028
Maubon T, Branger N, Bastide C, Lonjon G, Harvey-Bryan KA, Validire P, Giusiano S, Rossi D, Cathelineau X, Rozet F (2016) Impact of the extent of extraprostatic extension defined by Epstein’s method in patients with negative surgical margins and negative lymph node invasion. Prostate Cancer Prostatic Dis 19:317–321. https://doi.org/10.1038/pcan.2016.24
Kristiansen A, Drevin L, Delahunt B, Samaratunga H, Robinson D, Franck Lissbrant I, Stattin P, Egevad L (2017) Prognostic significance and biopsy characteristics of prostate cancer with seminal vesicle invasion on radical prostatectomy: a nationwide population-based study. Pathology 49:715–720. https://doi.org/10.1016/j.pathol.2017.08.008
Farris AB, Cynthia Cohen C, Rogers TE, Smith GH (2017) Whole slide imaging for analytical anatomic pathology and telepathology practical applications today, promises, and perils. Arch Pathol Lab Med 141:542–550
Goacher E, Randell R, Williams B, Treanor D (2017) The diagnostic concordance of whole slide imaging and light microscopy. A systematic review. Arch Pathol Lab Med 141:151–161
Montironi R, Cheng L, Lopez-Beltran A, Scarpelli M (2016) Quantitative image analysis on histologic virtual slides for prostate pathology diagnosis, response to chemopreventive agents, and prognosis. Eur Urol Focus 3:467–469. https://doi.org/10.1016/j.euf.2016.06.013
Montironi R, Cinmadamore A, Massari F, Montironi MA, Lopez-Beltran A, Cheng L, Montorsi F, Scarpelli M (2017) Whole slide imaging of large format histology in prostate pathology: potential for information fusion. Arch Pathol Lab Med 141:1460–1461
Pantanowitz L, Sinard JH, Henricks WH, Fatheree LA, Carter AB, Cintis L, Beckwith BA, Evans AJ, Otis CN, Lal A, Parwani AV (2013) Validating whole slide imaging for diagnostic purposes in pathology. Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med 137:1710–1722
Rodriguez-Urrego PA, Cronin AM, Al-Ahmadie HA, Gopalan A, Tickoo SK, Reuter VE, Fine SW (2011) Interobserver and intraobserver reproducibility in digital and routine microscopic assessment of prostate needle biopsies. Hum Pathol 42:68–74
Têtu B, Evans A (2014) Canadian licensure for the use of digital pathology for routine diagnoses one more step toward a new era of pathology practice without borders. Arch Pathol Lab Med 138:302–304
Danneman D, Wiklund F, Wiklund NP, Egevad L (2013) Prognostic significance of histopathological features of extraprostatic extension of prostate cancer. Histopathology 63:580–589. https://doi.org/10.1111/his.12199
Evans AJ, Henry PC, Van der Kwast TH, Tkachuk DC, Watson K, Lockwood GA, Fleshner NE, Cheung C, Belanger EC, Amin MB, Liliane B-G, Bostwick DG, Egevad L, Epstein JI, Grignon DJ, Jones EC, Montironi R, Moussa M, Sweet J, Trpkov K, Wheeler T, Srigley JR (2008) Interobserver variability between expert urologic pathologists for extraprostatic extension and surgical margin status in radical prostatectomy specimens. Am J Surg Pathol 32:1503–1512. https://doi.org/10.1097/PAS.0b013e31817fb3a0
Pierorazio PM, Walsh PC, Partin AW, Epstein JI (2013) Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 111(5):753–760. https://doi.org/10.1111/j.1464-410X.2012.11611.x
Epstein JI, Lars E, Amin MB, Delahunt B, Srigley JR, Peter H, the Grading Committee (2016) The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 40:244–252. https://doi.org/10.1097/PAS.0000000000000530
Epstein JI, Amin MB, Reuter VE, Humphrey PA (2017) Contemporary Gleason grading of prostatic carcinoma: an update with discussion on practical issues to implement the 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 41:e1–e7. https://doi.org/10.1097/PAS.0000000000000820
Humphrey PA, Amin MB, Berney DM, Billis A, Cao D, Cheng L, Delahunt B, Egevad L, Epstein JI, Fine SW, Grignon DJ, Christiansen G, Lopez-Beltran A, Magi-Galluzzi C, Netto GJ, Rubin MA, Samaratunga H, Srigley JR, True LD, Tsuzuki T, Van der Kwast T (2016) Acinar adenocarcinoma. In: Moch H, Humphrey PA, Ullbright TM, Reuter V (eds) WHO classification of tumours of the urinary system and male genital organs, 4th edn. IARC, Lyon, pp 138–162
Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, Dmochowski RR, Eton DT, Forman JD, Goldenberg SL, Hernandez J, Higano CS, Kraus SR, Moul JW, Tangen C, Trasher JB, Thompson I (2007) Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol 177(2):540–545. https://doi.org/10.1016/j.juro.2006.10.097
Author information
Authors and Affiliations
Contributions
ALB, MV, and VH conceived and designed the study and wrote, edited, and reviewed the manuscript. AB performed the statistical analysis and reviewed the manuscript. AC, MRR, NV, VH, FP, and ALB collected and analyzed data and reviewed the manuscript. RM and LC critically read and edited the final manuscript. All authors gave final approval for publication.
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Informed consent
Written informed consent was obtained from all patients included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Quality in Pathology
Rights and permissions
About this article
Cite this article
Volavšek, M., Henriques, V., Blanca, A. et al. Digital versus light microscopy assessment of extraprostatic extension in radical prostatectomy samples. Virchows Arch 475, 735–744 (2019). https://doi.org/10.1007/s00428-019-02666-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02666-x