Abstract
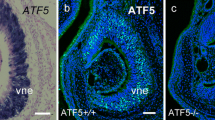
The accessory olfactory system controls social and sexual behaviours in mice, both of which are critical for their survival. Vomeronasal sensory neuron (VSN) axons form synapses with mitral cell dendrites in glomeruli of the accessory olfactory bulb (AOB). Axons of VSNs expressing the same vomeronasal receptor (VR) converge into multiple glomeruli within spatially conserved regions of the AOB. Here, we have examined the role of the cell adhesion molecule Kirrel2 in the formation of glomeruli within the AOB. We find that Kirrel2 expression is dispensable for early axonal guidance events, such as fasciculation of the vomeronasal tract and segregation of apical and basal VSN axons into the anterior and posterior regions of the AOB, but is necessary for glomeruli formation. Specific ablation of Kirrel2 expression in VSN axons results in the disorganization of the glomerular layer of the posterior AOB and in the formation of fewer and larger glomeruli. Furthermore, simultaneous ablation of Kirrel2 and Kirrel3 expression leads to a loss of morphologically identifiable glomeruli in the AOB, reduced excitatory synapse numbers, and larger presynaptic terminals. Taken together, our results demonstrate that Kirrel2 and Kirrel3 are essential for the formation of glomeruli and suggest they contribute to synaptogenesis in the AOB.







Similar content being viewed by others
References
Belluscio L, Koentges G, Axel R, Dulac C (1999) A map of pheromone receptor activation in the mammalian brain. Cell 97:209–220
Boschat C, Pélofi C, Randin O, Roppolo D, Lüscher C, Broillet MC, Rodriguez I (2002) Pheromone detection mediated by a V1r vomeronasal receptor. Nat Neurosci 5:1261–1262
Brignall AC, Cloutier J-F (2015) Neural map formation and sensory coding in the vomeronasal system. Cell Mol Life Sci 72:4697–4709
Chamero P, Katsoulidou V, Hendrix P, Bufe B, Roberts R, Matsunami H, Abramowitz J, Birnbaumer L, Zufall F, Leinders-Zufall T (2011) G protein Gαo is essential for vomeronasal function and aggressive behavior in mice. Proc Natl Acad Sci USA 108:12898–12903
Cho JH, Prince JE, Cutforth T, Cloutier JF (2011) The pattern of glomerular map formation defines responsiveness to aversive odorants in mice. J Neurosci 31:7920–7926
Cho JH, Kam JW, Cloutier JF (2012) Slits and Robo-2 regulate the coalescence of subsets of olfactory sensory neuron axons within the ventral region of the olfactory bulb. Dev Biol 371:269–279
Cloutier JF, Giger RJ, Koentges G, Dulac C, Kolodkin AL, Ginty DD (2002) Neuropilin-2 mediates axonal fasciculation, zonal segregation, but not axonal convergence, of primary accessory olfactory neurons. Neuron 33:877–892
Cloutier JF, Sahay A, Chang EC, Tessier-Lavigne M, Dulac C, Kolodkin AL, Ginty DD (2004) Differential requirements for semaphorin 3F and Slit-1 in axonal targeting, fasciculation, and segregation of olfactory sensory neuron projections. J Neurosci 24:9087–9096
Del Punta K, Puche A, Adams NC, Rodriguez I, Mombaerts P (2002) A divergent pattern of sensory axonal projections is rendered convergent by second-order neurons in the accessory olfactory bulb. Neuron 35:1057–1066
Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR (2001) Protreinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 21:4829–4836
Dulac C, Axel R (1995) A novel family of genes encoding putative pheromone receptors in mammals. Cell 83:195–206
Dulac C, Wagner S (2006) Genetic analysis of brain circuits underlying pheromone signaling. Annu Rev Genet 40:449–467
Eggan K, Baldwin K, Tackett M, Osborne J, Gogos J, Chess A, Axel R, Jaenisch R (2004) Mice cloned from olfactory sensory neurons. Nature 428:44–49
Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K (2010) The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature 466:118–122
Hammen GF, Turaga D, Holy TE, Meeks JP (2014) Functional organization of glomerular maps in the mouse accessory olfactory bulb. Nat Neurosci 17:953–961
Hasen NS, Gammie SC (2009) Trpc2 gene impacts on maternal aggression, accessory olfactory bulb anatomy and brain activity. Genes Brain Behav 8:639–649
Herrada G, Dulac C (1997) A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell 90:763–773
Jia C, Halpern M (1996) Subclasses of vomeronasal receptor neurons: differential expression of G proteins (Gi alpha2) and G(o alpha) and segregated projections to the accessory olfactory bulb. Brain Res 719:117–128
Juhila J, Lassila M, Roozendaal R, Lehtonen E, Messing M, Langer B, Kerjaschki D, Verbeek JS, Holthofer H (2010) Inducible nephrin transgene expression in podocytes rescues nephrin-deficient mice from perinatal death. Am J Pathol 176:51–63
Knoll B, Zarbalis K, Wurst W, Drescher U (2001) A role for the EphA family in the topographic targeting of vomeronasal axons. Development 128:895–906
Knoll B, Schmidt H, Andrews W, Guthrie S, Pini A, Sundaresan V, Drescher U (2003) On the topographic targeting of basal vomeronasal axons through Slit-mediated chemorepulsion. Development 130:5073–5082
Li M, Armelloni S, Ikehata M, Corbelli A, Pesaresi M, Calvaresi N, Giardino L, Mattinzoli D, Nisticò F, Andreoni S, Puliti A, Ravazzolo R, Forloni G, Messa P, Rastaldi MP (2011) Nephrin expression in adult rodent nervous system and its interaction with glutamate receptors. J Pathol 225:118–128
Martin EA, Muralidhar S, Wang Z, Cervantes DC, Basu R, Taylor MR, Hunter J, Cutforth T, Wilke SA, Ghosh A, Williams ME (2015) The intellectual disability gene Kirrel3 regulates target-specific mossy fiber synapse development in the hippocampus. Elife 4:e09395. doi:10.7554/eLife.09395
Martini S, Silvotti L, Shirazi A, Ryba NJ, Tirindelli R (2001) Co-expression of putative pheromone receptors in the sensory neurons of the vomeronasal organ. J Neurosci 21:843–848
Matsunami H, Buck LB (1997) A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell 90:775–784
Morikawa Y, Komori T, Hisaoka T, Ueno H, Kitamura T, Senba E (2007) Expression of mKirre in the developing sensory pathways: its close apposition to nephrin-expressing cells. Neurosci 150:880–886
Pantages E, Dulac C (2000) A novel family of candidate pheromone receptors in mammals. Neuron 28:835–845
Papes F, Logan DW, Stowers L (2010) The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell 141:692–703
Pavenstadt H, Kriz W, Kretzler M (2003) Cell biology of the glomerular pedocyte. Physiol Rev 83:253–307
Prince JE, Cho JH, Dumontier E, Andrews W, Cutforth T, Tessier-Lavigne M, Parnavelas J, Cloutier JF (2009) Robo-2 controls the segregation of a portion of basal vomeronasal sensory neuron axons to the posterior region of the accessory olfactory bulb. J Neurosci 29:14211–14222
Prince JEA, Brignall AK, Cutforth T, Shen K, Cloutier J-F (2013) Kirrel-3 is required for the coalescence of vomeronasal sensory neuron axons into glomeruli and for male-male aggression. Development 140:2398–2408
Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K (2001) The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10:1–8
Rodriguez I, Feinstein P, Mombaerts P (1999) Variable patterns of axonal projections of sensory neurons in the mouse vomeronasal system. Cell 97:199–208
Rodriguez I, Del Punta K, Rothman A, Ishii T, Mombaerts P (2002) Multiple new and isolated families within the mouse superfamily of V1r vomeronasal receptors. Nat Neurosci 5:134–140
Ryba NJ, Tirindelli R (1997) A new multigene family of putative pheromone receptors. Neuron 19:371–379
Serizawa S, Miyamichi K, Takeuchi H, Yamagishi Y, Suzuki M, Sakano H (2006) A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell 127:1057–1069
Shen K, Bargmann CI (2003) The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell 112:619–630
Shen K, Fetter RD, Bargmann CI (2004) Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell 116:869–881
Stowers L, Logan DW (2010) Olfactory mechanisms of stereotyped behavior: on the scent of specialized circuits. Curr Opin Neurobiol 20:274–280
Walz A, Rodriguez I, Mombaerts P (2002) Aberrant sensory innervation of the olfactory bulb in neuropilin-2 mutant mice. J Neurosci 22:4025–4035
Walz A, Feinstein P, Khan M, Mombaerts P (2007) Axonal wiring of guanylate cyclase-D-expressing olfactory neurons is dependent on neuropilin 2 and semaphorin 3F. Development 134:4063–4072
Zufall F, Leinders-Zufall T (2007) Mammalian pheromone sensing. Curr Opin Neurobiol 17:483–489
Acknowledgements
We thank members of the Cloutier lab for helpful discussion and technical advice. We thank Drs. Louis Hermo and Thomas Stroh, as well as the Facility for Electron Microscopy Research of McGill University for invaluable help and advice with synapse analyses. We thank Dr. David Ginty for Kirrel cDNAs and Mitra Cowan for the generation of Kirrel2 mouse models. This work was supported by the Canadian Institutes for Health Research and the Natural Sciences and Engineering Council of Canada (J.-F. C.). A. C. B held a studentship from the Natural Sciences and Engineering Council of Canada. J. E. A. Prince held studentships from the Fonds Québécois pour la Recherche sur la Nature et les Technologies and the Faculty of Medicine at McGill University. Reesha Raja is a recipient of a Vanier Scholarship from the Canadian Institutes of Health Research. J.-F. C. is a FRQS Chercheur Sénior.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brignall, A.C., Raja, R., Phen, A. et al. Loss of Kirrel family members alters glomerular structure and synapse numbers in the accessory olfactory bulb. Brain Struct Funct 223, 307–319 (2018). https://doi.org/10.1007/s00429-017-1485-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1485-0