Abstract
Congenital cytomegalovirus (CMV) infection leads to olfactory bulb lesions in the fetus, yet little is known about its impact on olfaction after birth. Here, we have assessed in a prospective study conducted on children in two French hospitals from 2016 to 2019, infection severity and olfactory performance after congenital CMV infection. Children with congenital CMV infection aged 3 to 10 years and healthy controls (CTL) matched for age and sex to CMV children symptomatic at birth (sCMV) were enrolled. Olfactory discrimination was assessed using mono-odorants and binary mixtures. Data were analyzed for 54 children with PCR-confirmed congenital CMV infection, including 34 sCMV (median [IQR] age, 6 [5–8] years; 19 [55.9%] male), and 20 CMV asymptomatic at birth (aCMV, median [IQR] age, 4 [3–6] years; 12 [60.0%] male). sCMV were compared to 34 CTL children. Olfactory scores in CMV-infected children were independent from vestibular deficit and hearing loss. The olfactory score was efficient to discriminate between CTL and sCMV for children > 6 years (area under the receiver-operating characteristic curve (AUC, 0.85; P = 0.0006), but not for children < 7 years. For children > 6 years, the proportion of children with total olfactory score < 4 differed between sCMV and CTL groups (91.2% and 18.7%, P < 0.001), but not between aCMV and age-matched healthy control groups.
Conclusion: Congenital CMV infection is associated with reduced olfactory performance in children with infection symptoms at birth.
Clinical trial registration: NCT02782988 (registration date: May 26, 2016).
What is Known: •Congenital cytomegalovirus infection leads to olfactory bulb lesions in the fetus, yet little is known about its impact on olfaction after birth. •Depending on neonatal clinical presentation, children are either categorized as having a symptomatic or asymptomatic infection at birth. | |
What is New: •Congenital cytomegalovirus infection is associated with reduced olfactory performance in children with infection symptoms at birth. |
Similar content being viewed by others
Introduction
Cytomegalovirus (CMV) is a herpes type 5 virus that can affect the fetal and neonatal brain after in utero infection [1]. CMV affects 0.5–2% of newborns and is the leading infectious cause of congenital deafness. Depending on neonatal clinical presentation, children are either categorized as having a symptomatic (sCMV) (presenting with growth retardation, prematurity, jaundice, petechiae, liver, and/or hematological anomalies) or asymptomatic (aCMV) (no clinical sign of infection other than possible hearing loss) infection at birth. Prognostic factors for neurosensory sequelae comprise gestational age at infection and sCMV [2, 3]. A total of 40–60% of sCMV and 10–20% of aCMV children will manifest varying degrees of hearing loss, which can be present at birth or may occur in the first months or years [4]. Although 90% of clinical presentations are silent at birth, no systematic newborn screening has been established to identify aCMV children who are at risk of hearing loss. Human CMV has a specific olfactory receptor expressed on olfactory neurons in the olfactory system that may define viral olfactory cell tropism [5]. Congenital CMV exhibits tropism for neural stem cells of the olfactory system of fetuses, thus lesioning the olfactory bulb [6,7,8]. This infection leads to both olfactory and hearing impairments in a mouse model [9]. However, little is known about olfactory dysfunction in CMV-infected children, partly because it is challenging to assess olfaction in toddlers. Many studies have shown the difficulty to reliably test children under 5 years [10,11,12,13] because of the cognitive and verbal involvement. Discrimination tasks are the most relevant because they are rapid to perform, unlike threshold tasks, and they are requiring minimal cognitive and verbal skills, contrary to identification tasks. New tests based on perception level could constitute useful tools to address olfaction in children. In this regard, mixture based olfactory discrimination tests perform better than standard smell tests in adult humans and in adult and pup animal models [9, 14]. Here, we report the olfactory performance of children with a confirmed congenital CMV infection, using a new psychophysical test we have developed. This test aims at measuring the discrimination of monomolecular odorants from the Sniffin’ test battery [15] and the discrimination of mixture odorants presented in Sniffin’ pens. It is non-invasive and rapid to perform, even in very young children, thus requiring little attention and concentration.
Methods
Study overview and ethical considerations
The main objective of this study was to investigate the association between hearing loss and olfactory performance in children with a congenital CMV infection followed in Robert Debré (Paris) and Bicêtre (Le Kremlin- Bicêtre) hospitals, in France. This prospective study is a nontrial, nondrug study, qualified as exploratory, multicenter, in a paediatric population (ClinicalTrials.gov number, NCT02782988). It received ethical approval (No. 3372) from Comité de Protection des Personnes (CPP IDF-3). Children were included in the study after explanation of the study and obtaining of written informed consent from both parents.
Enrolment criteria
Children with confirmed congenital CMV, aged 3 to 10 years, were enrolled in this study during a standard care visit. Proof of congenital infection was ascertained by positive CMV polymerase chain reaction (PCR) in urine and/or blood in the first 3 postnatal weeks, or retrospective diagnosis for the presence of positive PCR on dried blood spots collected at postnatal days 3 to 7.
Exclusion criteria included clinical conditions that may interfere with the study, such as chronic rhinosinusitis, allergic rhinitis, primary ciliary dyskinesia, Kallmans syndrome, or other neurologic issues that can impact olfaction.
CMV infected children were divided into two groups according to neonatal characteristics consistent with recognized clinical definitions: sCMV and aCMV at birth. Healthy controls (CTL) matched for age and sex to the sCMV group were enrolled among children consulting for other ear, nose, throat (ENT) non-rhinological pathologies, anaesthesiology, or orthopedic appointments. CTL children had no history of congenital infection and presented with transient evoked otoacoustic emissions < 20 dB for each ear.
Clinical and radiologic symptoms
Prenatal and neonatal clinical signs and virological data in favor of congenital CMV infection were recorded. Postural developmental milestones, vestibular canal, and otolithic function were assessed as previously described [16]. Magnetic resonance imaging of the brain and the inner ear was performed to assess cerebral lesions (see the Supplemental Information for details).
Hearing evaluation
Children with congenital CMV underwent either objective auditory brainstem response or subjective behavioral audiometry tests to assess auditory thresholds. Hearing deficit was defined by an auditory threshold of the most affected ear ≥ 25 dB. In CTL, normality of hearing was assessed using evoked otoacoustic emissions.
Olfactory evaluation
Olfaction was assessed in a 15-min session with 18 pen-like odor-dispensing devices (Sniffin’ Sticks, Burghardt, Wedel, Germany) [15]. Two series of 3-odorant discrimination tasks were performed: the first with simple odorants (monomolecular odorant test) and the second with binary mixtures of odorants (mixture odorant test). For each task, 3 Sniffin’ Sticks were sequentially presented to the subject, two contained the same odorant and one contained a different associated odorant. The child was requested to smell each stick and indicate the stick that smells differently (forced choice between three possibilities). A correct or incorrect answer resulted in a score of 1 or 0, respectively.
Monomolecular Odorant Test
The sticks for the first task contained isoamylacetate (one stick) and anethol odorant (two sticks). The sticks for the second task contained limonene (one stick) and citronellal odorant (two sticks). The sticks for the third task contained anethol (one stick) and eugenol odorant (two sticks). The total score for this test ranged from 0 (no correct response) to 3 (all correct responses). Binary variables were defined using the threshold of 2.
Mixture odorant test
The sticks for the first task contained a mixture of L-carvone and D-carvone at a 2:8 proportion (one stick) and mixture of L-carvone and D-carvone at an 8:2 proportion (two sticks). The sticks for the second task contain a mixture of isoamylacetate and anethol in an 8:2 proportion (one stick) and mix of isoamylacetate and anethol at a 2:8 proportion (two sticks). The sticks for the third task contain a mixture of anethol and eugenol at an 8:2 proportion (one stick) and mix of anethol and eugenol at a 2:8 proportion (two sticks). The total score for this test ranged from 0 (no correct response for the 3 problems) to 3 (correct responses for the 3 problems). Again, binary variables were defined using the threshold of 2.
Olfactory score calculation
The total olfactory score (TOS) was calculated by adding the monomolecular odorant score to the mixture score. It ranged from 0 (no correct response for the 6 problems) to 6 (correct responses for the 6 problems). Binary variables were defined by a total score < 4; this threshold was retained as it corresponds to a majority of incorrect responses.
Statistical analysis
Quantitative variables were summarized as median with interquartile range (IQR) and compared across groups using Mann–Whitney non-parametric test. Categorical data were expressed as percentages and compared between groups using Fisher exact test. The accuracy of olfactory tests was evaluated by applying data to receiver-operating characteristic (ROC) curves. To study the associations between children characteristics and olfaction, the Spearman non-parametric test was used. Statistical analyses were performed using Stata 16 (StataCorp LLC, Texas, USA) and Prism software (GraphPad, version 9, San Diego, USA), significance was considered at the level 5%.
Results
Children characteristics
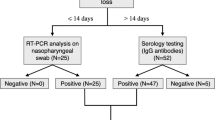
From May 2016 to December 2019, we recruited 34 sCMV children (median [IQR] age, 6 [5,6,7,8] years; 19 [55.9%] male; Tables 1, S1 and S2). We also recruited 34 healthy matched-CTL. As a supplementary control, we included aCMV children. However, due to the absence of CMV newborn screening in France, enrolment of aCMV was complex, particularly in the 7–10-year age group, and only 20 aCMV were enrolled (median [IQR] age, 4 [3,4,5,6] years (only 5 children aged 7–10); 12 [60.0%] male. Thus, we ultimately essentially compared sCMV to CTL children because we did not reach the targeted number of aCMV children. Figure 1 shows the flow chart of the selection process.
Among the 54 children with congenital CMV infection, 23 presented hearing or vestibular deficit at inclusion. Hearing deficits were reported in 19 children (12 in the sCMV group and 7 in the aCMV group). Three presented with profound congenital hearing loss at birth (1 in the sCMV group and 2 in the aCMV group).
Olfactory performance
Among CTL, both the monomolecular odorant discrimination score and the TOS were positively correlated with age (r = 0.42, P = 0.012; and r = 0.48, P = 0.004, respectively). In CTL, TOS was significantly higher in children 7–10 years than in those 3–6 years (median (IQR): 4.0 [4.0–5.0] and 3.0 [1.0–4.0], P = 0.002), and in consequence, the proportion with a TOS < 4 was significantly lower in CTL 7–10 years than in CTL 3–6 years (18.75% and 66.7%, respectively; P = 0.007; Table 2). Considering the monomolecular odorant discrimination score, the proportion with a score < 2 was significantly lower in controls aged 7–10 years than in controls aged 3–6 years (6.3% and 55.6%, respectively; P = 0.003). Considering the mixture odorant discrimination score, the proportion with a score < 2 was not different between CTL aged 7–10 and 3–6 years (37.5% and 61.1%, respectively; P = 0.30). There was no association between olfactory scores and sex or with passive smoking.
ROC curve analysis revealed that the TOS was efficient to discriminate between CTL and sCMV for children 7–10 years (area under the ROC curve [AUC] = 0.857, P = 0.0006; Fig. 2b), but not for children 3–6 years (AUC = 0.519; Fig. 2a). Moreover, for children > 6 years, the mixture score alone was efficient to discriminate between CTL and sCMV (AUC = 0.809, P = 0.003; Fig. 2d), but not the monomolecular odorant score (AUC = 0.588; Fig. 2c).
ROC curves for the discrimination of children with congenital cytomegalovirus infection and controls using the olfactory scores. Panels a–d show the ROC curves for the discrimination of sCMV and matched controls between 3–6 years (a) and 7–10 years (b–d) using the olfactory score (a, b), the monomolecular odorant score (c), and the mixture score (d). N = 34 sCMV; N = 34 CTL
Overall, the proportion of children with a TOS < 4 was significantly higher in the sCMV group than in the CTL group (73.5% and 44.1%; P = 0.025). Considering only the monomolecular odorant discrimination score, there was no difference between the two groups (Fig. 3b). For the only mixture scores, the proportion of children with a score < 2 was significantly higher in the sCMV group than in the CTL group (76.5% and 50.0%, respectively, P = 0.043).
Olfactory scores in children with congenital cytomegalovirus infection and controls. Panels a–e show the total olfactory score (a, d, e), the monomolecular odorant (b), and mixture (c) scores. Box and whiskers showing median, 10 percentile, 25 percentile, 75 percentile, and 90 percentile in bar graphs. P < 0.05 are shown. N = 54 CMV including 34 sCMV and 20 aCMV. N = 34 CTL
Stratifying by age, the difference in the proportion of children with a TOS < 4 was highly significant between sCMV and CTL in children 7–10 years of age (91.2% and 18.7%, P < 0.001), but not in younger children (Fig. 3d).
In sCMV children, there was no difference in the TOS between children presenting with and those without neurological involvement (Fig. S1). There was no difference for the TOS between sCMV children presenting with hearing loss and those with normal hearing (Fig. 3e).
There was no difference in the proportion of children with a TOS < 4 between aCMV, subset of age-matched sCMV, and subset of age-matched CTL children in the 7–10-year age group as well as in younger children (Fig. S2).
There was no difference in the olfactory scores between children who received antiviral treatment after CMV detection (n = 7) and those without treatment (n = 38) (Table 2).
Discussion
This is the first study to assess olfactory function in children with congenital CMV infection and to report the severity of their altered olfaction ability. The strengths of this study are (i) PCR-confirmed congenital CMV infection; (ii) the documentation of clinical, radiologic, and vestibular symptoms as well as concomitant evaluation of hearing; and (iii) enrolment of age- and sex-matched CTL.
Reduced olfactory score was frequent in congenital CMV infection, occurring in 91.2% of our sCMV patients aged 7–10 years, thus becoming the most frequent sensorineural deficit in our series. A total of 44.1% of these patients experience other sensorineural deficits (hearing loss in 35.3%, vestibular deficit in 38.2%). Conversely, 5 aCMV children aged 7–10 years demonstrated normal olfaction. The most likely explanation of this observation is the probable link between olfactory performance and the severity of congenital CMV infection. A recent retrospective study demonstrated that 67% of children with olfactory dysfunction were of congenital origin, whereas 12% were due to head trauma [13]; the role of congenital infection being to date unknown, the responsibility of CMV has certainly not yet been evaluated. In previous studies, loss of smell in infants has been linked to neurodevelopmental disorders, including attention deficit/hyperactivity disorders and autism spectrum [10, 17]. Olfaction is essential for food information, safety, emotion regulation, scaffolds environment perception and memory, mother–child attachment, and social cognition [18]. However, there is no absolute correlation between neurodevelopmental disorders and olfactory scores, as we do not find a link between these two in our present series.
Olfactory loss can also be observed after other post-viral infections such as rhinovirus, parainfluenza virus, coronavirus (CoV) 229E, and Epstein-Barr virus [19]. Olfactory discrimination and thresholds were preserved in these latter infections, compared to identification [20]. Olfactory loss can be an early sign of coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome CoV-2; this dysfunction can persist several months and be associated to an olfactory bulb hypometabolism [21,22,23]. Fetopathological studies have demonstrated the presence of CMV in neural stem cells of the olfactory bulb underlining the specific targeting of the pluripotent cells, rather than olfactory neurons [8].
Olfactory scores in our CMV-infected children were independent from age, contrasting with CTL children. Improved olfactory performance in healthy children is correlated with the maturation of the olfactory system with better ability to discriminate with age. This is not observed in sCMV-infected children, possibly due to the viral targeting of pluripotent cells [8]. Olfactory scores in our CMV-infected children were independent from hearing loss or vestibular deficit. These findings contrast with an epidemiological study where a correlation was found between hearing loss and olfactory dysfunction, but infection, in particular congenital, was not considered as an influential factor [24]. The incidence of cranial neuropathies is higher in patients with post-viral olfactory loss compared to a control population [25]; however, we found no difference for the olfactory score between children presenting neurological manifestations and those without neurological involvement. These findings suggest that peripheral (audiovestibular) and central (cerebral) lesions are independent and that neurological damage did not induce vulnerability to olfactory dysfunction in our sCMV infants. CMV host entry is probably systemic, associated with macrophage infection [26]. To date, there is no evidence of CMV spread to the brain through the cribriform plate.
Another insight of our study is the greater efficiency of the mixture discrimination tests in assessing olfactory function in children compared to the mono-odorant testing. While the monomolecular test evaluates the ability to discriminate between two single odorants of similar concentration, the mixture test is a more difficult perception test with discrimination of mixtures presenting the same two odorants but in different concentration. Of note, the odorant mixture discrimination score only discriminates between CTL and CMV from the age of 7, which strongly limits its use in clinics. The lower discrimination efficacy in younger children may be due to the subtler olfactory difference between scent pens that children 3–6 may be less attentive to.
Limitations of our study include the use of olfactory tests that have not been validated for children in this version before and a predefined cutoff value that was not based on previous observations in a control group. The cutoff value first appears in the initial statistical plan of the study’s protocol, that was subject to no change. This cutoff of 4 points to distinguish between normosmia and olfactory dysfunction was retained in the initial statistical plan of this study as it corresponds to a majority of incorrect responses. This cutoff leads to a high percentage of children in the control group with reduced olfactory function. Another limit of our study is the small sample size of the human cohort, especially for aCMV patients. Extending these investigations to a larger group of children, including controls, would allow specifying these first findings. Moreover, this study would benefit from additional approaches to characterize the olfactory function, by using tests of perception and identification of odorants.
In conclusion, this study highlighted the high incidence of olfactory impairment in children with congenital sCMV infection. As olfactory loss can impact nutrition, social interaction, safety and quality of life, early detection of olfactory disorders may lead to olfactory rehabilitation programs in order to limit neurodevelopmental consequences: recent studies have demonstrated the importance of olfactory training to improve the olfactory function in adults [27, 28] and children [29].
Data availability
Some deidentified individual participant data will be made available from the corresponding author, upon reasonable request. The data are not publicly available because they contain information that could compromise the privacy of our patients.
Code availability
Not applicable.
Abbreviations
- aCMV:
-
Asymptomatic cytomegalovirus infection at birth
- AUC:
-
Area under the ROC curve
- CMV:
-
Cytomegalovirus
- CoV:
-
Coronavirus
- COVID-19:
-
Coronavirus disease 2019
- CTL:
-
Control
- ENT:
-
Ear, nose, throat
- IQR:
-
Interquartile range
- PCR:
-
Polymerase chain reaction
- ROC:
-
Receiver-operating characteristic
- sCMV:
-
Symptomatic cytomegalovirus infection at birth
- TOS:
-
Total olfactory score
References
Coyne CB, Lazear HM (2016) Zika virus — reigniting the TORCH. Nat Rev Microbiol 14(11):707–715
Nicloux M, Peterman L, Parodi M, Magny JF (2020) Outcome and management of newborns with congenital cytomegalovirus infection. Arch Pediatr 7(3):160–165
Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF (2000) Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol 11(5):283–290
Goderis J, Keymeulen A, Smets K et al (2016) Hearing in children with congenital cytomegalovirus infection: results of a longitudinal study. J Pediatr 172:110-115.e2
E X, Meraner P, Lu P, et al (2019) OR14I1 is a receptor for the human cytomegalovirus pentameric complex and defines viral epithelial cell tropism. Proc Natl Acad Sci U S A 116(14):7043–7052
van Den Pol AN, Mocarski E, Saederup N et al (1999) Cytomegalovirus cell tropism, replication, and gene transfer in brain. J Neurosci 19(24):10948–10965
Odeberg J, Wolmer N, Falci S, Westgren M, Seiger A, Söderberg-Nauclér C (2006) Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J Virol 80(18):8929–8939
Teissier N, Fallet-Bianco C, Delezoide AL et al (2014) Cytomegalovirus-induced brain malformations in fetuses. J Neuropathol Exp Neurol 73(2):143–158
Lazarini F, Katsimpardi L, Levivien S et al (2018) Congenital cytomegalovirus infection alters olfaction prior to hearing deterioration in mice. J Neurosci 38(49):10424–10437
Doty RL (2001) Olfaction. Annu Rev Psychol 52:423–452
Hummel T, Bensafi M, Nikolaus J et al (2007) Olfactory function in children assessed with psychophysical and electrophysiological techniques. Behav Brain Res 180(2):133–138
Cameron EL, Doty RL (2013) Odor identification testing in children and young adults using the smell wheel. Int J Pediatr Otorhinolaryngol 77(3):346–350
Schriever VA, Hummel T (2020) Etiologies of olfactory dysfunction in a pediatric population: based on a retrospective analysis of data from an outpatient clinic. Eur Arch Otorhinolaryngol 277(11):3213–3216
Hsieh JW, Keller A, Wong M, Jiang RS, Vosshall LB (2017) SMELL-S and SMELL-R: Olfactory tests not influenced by odor-specific insensitivity or prior olfactory experience. Proc Natl Acad Sci U S A 114(43):11275–11284
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) “Sniffin” sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22(1):39–52
Maudoux A, Teissier N, Francois M et al (2020) Vestibular impact of Friedreich ataxia in early onset patients. Cerebellum Ataxias 7:6
Rozenkrantz L, Zachor D, Heller I et al (2015) A mechanistic link between olfaction and autism spectrum disorder. Curr Biol 25(14):1904–1910
Schaal B, Saxton TK, Loos H et al (2020) Olfaction scaffolds the developing human from neonate to adolescent and beyond. Phil Trans R Soc B 375(1800):20190261
Suzuki M, Koichi Saito K, Min W-P et al (2007) Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 117(2):272–277
Whitcroft KL, Cuevas M, Haehner A et al (2017) Patterns of olfactory impairment reflect underlying disease etiology. Laryngoscope 127(2):291–295
Niklassen AS, Draf J, Huart C et al (2021) COVID-19: Recovery from chemosensory dysfunction. A multicentre study on smell and taste. Laryngoscope 131(5):1095–1100
de Melo GD, Lazarini F, Levallois S et al (2021) COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med 13(596):eabf8396
Guedj E, Lazarini F, Morbelli S et al (2021) Long COVID and the brain network of Proust’s madeleine: targeting the olfactory pathway. Clin Microbiol Infect S1198–743X(21)00238-X
Park JH, Byeon HK, Park KN et al (2017) Epidemiological association of olfactory dysfunction with hearing loss and dysphonia in the Korean population: a cross-sectional study. Medicine (Baltimore) 96(47):e8890
Jitaroon K, Wangworawut Y, Ma Y, Patel ZM (2020) Evaluation of the incidence of other cranial neuropathies in patients with postviral olfactory loss. JAMA Otolaryngol Head Neck Surg 146(5):465–470
Farrell HE, Stevenson PG (2019) Cytomegalovirus host entry and spread. J Gen Virol 100(4):545–553
Addison AB, Wong B, Ahmed T et al (2021) Clinical olfactory working group consensus statement on the treatment of post infectious olfactory dysfunction. J Allergy Clin Immunol 147(5):1704–1719
Zhang Y, Mei T, Chen Y et al (2021) Smell disorders in COVID-19 patients: role of olfactory training: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 100(8):e24862
Mahmut MK, Pieniak M, Resler K et al (2021) Olfactory training in 8-year-olds increases odour identification ability: a preliminary study. Eur J Pediatr 180(7):2049–2053
Acknowledgements
We thank the children who participated in this study, all the physicians, and clinical research assistants who helped conduct this study. We thank Kurt Sailor, Perception and Memory Laboratory, Institut Pasteur, Paris, France, for his critical review of the manuscript. We also thank Nathalie Jolly and Olivia Chény, Clinical Core, CRT, Institut Pasteur, Paris, France.
Funding
This study was supported by Institut Pasteur of Paris (GPF 2015 Microbes and Brain “INFECSMELL”), Inserm, and Université de Paris and sponsored by Institut Pasteur of Paris (2015–091).
Author information
Authors and Affiliations
Contributions
Françoise Lazarini, Pierre-Marie Lledo, and Natacha Teissier contributed to the study conception and design. Material preparation and data collection were performed by Sarah Levivien, and Natacha Teissier. Estelle Mottez and Tan-Phuc Buivan provided administrative and technical support. Interpretation of data and analysis were performed by Françoise Lazarini, Sarah Levivien, Audrey Maudoux, Sylvette Wiener-Vacher, Jerome Nevoux, Thierry Van Den Abbeele, Pierre-Gressens, Pierre-Marie Lledo, and Natacha Teissier. Yoann Madec and Fabien Taieb supervised the statistical analysis. The first draft of the manuscript was written by Françoise Lazarini, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the declaration of Helsinki. Ethics approval (No. 3372) was received from Comité de Protection des Personnes (CPP IDF-3). All participants gave consent prior to beginning the study.
Consent for publication
All authors named consent to the publication of this manuscript.
Competing interests
The odorant mixtures are the subject of a patent (WO2017198816A1 published on November 23, 2017) by Institut Pasteur, Centre National de la Recherche Scientifique, and Assistance Publique–Hôpitaux de Paris on which Drs Lazarini, Lledo, Teissier, and Levivien are named as inventors. Drs Lazarini, Madec, Taieb, Mottez, Lledo, and Mr Buivan are employees of Institut Pasteur of Paris that sponsored this research. The remaining authors declare no other disclosures.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lazarini, F., Levivien, S., Madec, Y. et al. Olfactory function in congenital cytomegalovirus infection: a prospective study. Eur J Pediatr 181, 1859–1869 (2022). https://doi.org/10.1007/s00431-022-04375-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04375-1