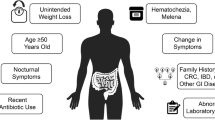
Abstract
Disorders of the gut-brain interaction negatively impact quality of life and carry a substantial socioeconomic burden. Irritable bowel syndrome (IBS) and functional abdominal pain-not otherwise specified (FAP-NOS) are common functional abdominal pain disorders in childhood. The pathophysiology is not fully understood, and high-quality intervention trials and international guidelines are missing. Therefore, the management of these disorders remains challenging. This review aims to provide an up-to-date overview of therapeutic possibilities for pediatric IBS or FAP-NOS and recommends management strategies. To prevent unnecessary referrals and extensive costs, it is fundamental to make a positive diagnosis of IBS or FAP-NOS in children with chronic abdominal pain with only minimal investigations. A tailor-made approach for each patient, based on the accompanying physical and psychological symptoms, is proposed to date.
Conclusion: Shared decision-making including non-pharmacological and pharmacological interventions should be considered and discussed with the family.
What is Known: • Irritable bowel syndrome and functional abdominal pain-not otherwise specified are common in childhood. • Although the number of treatment options has grown recently, managing these disorders can be challenging and unsatisfactory, and no evidence-based international management guidelines are available. | |
What is New: • We suggest using a stepwise individualized approach to management, where after first-line management, both non-pharmacological and pharmacological interventions should be discussed. |
Similar content being viewed by others
Introduction
Functional abdominal pain disorders (FAPDs) are disorders of the gut-brain axis characterized by chronic abdominal pain and altered bowel movements in the case of irritable bowel syndrome (IBS). FAPDs comprise four disorders: functional dyspepsia, IBS, abdominal migraine, and functional abdominal pain-not otherwise specified (FAP-NOS) (Supplemental Table 1) [1, 2]. In IBS, four types can be distinguished: predominant-diarrhea (IBS-D), predominant-constipation (IBS-C), mixed or alternating stool forms (IBS-A), and unclassified (IBS-U) [3].
FAPDs are common, with an estimated prevalence ranging from 1.6 to 41.2% in the pediatric population. These disorders have a profoundly negative impact on quality of life and carry a substantial socioeconomic burden [4,5,6]. Despite their high prevalence and impact, the pathophysiology underlying FAPDs is not well understood. FAPDs are presumably multifactorial and include genetic factors; psychological factors such as child abuse, stress, or depression; hypersensitivity to food products; and gut microbiota alterations [7]. Although the number of treatment options has grown recently, managing these disorders can be challenging and unsatisfactory. In this review, we aim to provide an up-to-date overview of therapeutic approaches, and we recommend management strategies focusing on pediatric IBS and FAP-NOS.
Methods
We searched for relevant articles in English up to August 2021 in PubMed, MEDLINE, EMBASE, PsycINFO, and Cochrane Library. To identify unpublished or ongoing studies, the ClinicalTrials.gov register, the Current Controlled Trials meta-Register of Controlled Trials–active registers, and the WHO International Clinical Trials Registry Platform Search Portal were searched. To identify relevant articles and reviews missed by the search strategies, the reference lists from reviewed articles were searched by hand. Only randomized controlled trials and systematic reviews were included. The full search strategies are available upon request. Inclusion and exclusion criteria are presented in Supplementary File 1. We assessed the risk of bias of all included studies in earlier studies, using the Cochrane risk of bias tool [8,9,10,11,12].
Management of IBS and FAP-NOS
Treatment often includes one or more of these strategies: (1) first-line management consisting of validation, explanation, and a positive diagnosis, (2) non-pharmacological treatment, and (3) pharmacological treatment.
-
1.
First-line management
The cornerstone of helping a child with IBS or FAP-NOS is first to validate the symptoms followed by a proper explanation of the diagnosis according to the biopsychosocial model [7]. An evidence-based, multidisciplinary treatment plan is essential to improve recovery and long-term prognosis [13].
-
Validation, explanation, and a positive diagnosis. One of the first steps is to acknowledge that the pain is real even though no severe organ damage is present. It can be helpful to explain that the pain is caused by hypersensitive nerves, using metaphors like a fire alarm that keeps on alarming although there is no fire [13]. Enough time must be allocated to make a positive diagnosis by discussing all the evidence that supports your diagnosis of IBS or FAP-NOS. Education on the interplay of different biopsychosocial factors that generate and maintain chronic abdominal complaints is also helpful. Finally, one needs to elicit expectations and elucidate that the long-term prognosis is favorable. The primary treatment goal should not be the complete eradication of pain but optimization of daily functioning, including school participation, a normal sleep pattern, and participation in extracurricular activities [14, 15]. The practitioner should remain connected with patients and parents through email and/or phone contact and follow-up visits tailored to each case every 4–12 weeks to increase treatment adherence and reduce the feeling that patients and families are discharged and left without support.
-
The parental response to their child’s abdominal pain. A multidisciplinary family approach is an essential part of the treatment strategy. An RCT studied the effects of parental attention versus distraction versus no instruction in children with chronic FAP [16]. Abdominal complaints were reduced by half in the distraction group and nearly doubled in the attention group. The study suggests that parental distraction is a powerful coping strategy. Moreover, Lindley et al. showed that healthcare consumerism in families lacking insight into their child’s problem can be harmful to the child with FAP [17]. Prognostic indicators of “healthcare consumerism” were refusal to engage with psychological services, involvement of more than three consultants, lodging of a manipulative complaint with hospital management by the child’s family, and lack of development of insight into psychosocial influences on symptoms [17].
-
Identify psychological and physical stressors that may play a crucial role in a child’s abdominal pain experience and, possibly, help reverse them. Parental acceptance of the biopsychosocial model of illness has shown to be an important factor for symptom relief in children with FAPDs [18].
-
Additional analgesic therapysuch as non-steroidal anti-inflammatory drugs, acetaminophen, and aspirin is sometimes used by general practitioners to treat pain. However, the efficacy of these drugs in treating pediatric chronic abdominal pain is not supported in any clinical trial and should be used with caution in clinical practice [19,20,21].
-
-
2.
Non-pharmacological treatment
Dietary interventions
In the last decade, there has been a great interest in the role of diet in the pathogenesis and management of FAPDs. More than 90% of children with a FAPD report that at least one food is associated with deterioration of their GI symptoms. As a result, children frequently avoid foods and implement diet strategies [22, 23]. However, it is likely that these food-associated symptoms are more the result of the gastrocolic reflex than that they are caused by food intolerances [24,25,26]. Indeed, research has shown little evidence that dietary interventions are helpful for this population [15, 27, 28]. There is some evidence regarding probiotics and dietary fibers, such as psyllium fibers [9, 12]. A detrimental effect of gluten is frequently self-reported [29]. Non-celiac gluten sensitivity is a clinical condition that has been insufficiently studied in children but may contribute to trigger or worsen GI symptoms (Table 1) [30].
Dietary fiber
A normal fiber intake is recommended for every child [31, 32]. Inadequate fiber intake has been proposed as a risk factor for developing FAPDs in children [33, 34]. Increasing dietary fiber intake was recommended as first-line treatment for IBS since fibers potentially decrease intracolonic pressure, accelerate gut transit time, and reduce abdominal pain [35, 36]. Soluble fibers may be particularly useful in the management of IBS-C, since they attract water into stools and therefore may relieve symptoms of constipation [37, 38]. However, increased gas production may also occur due to fiber fermentation [39]. A meta-analysis of adult studies has shown the benefit of soluble fibers, such as psyllium, as opposed to insoluble fiber, such as bran [40, 41]. Therefore, adult IBS clinical guidelines support soluble fiber in IBS treatment [39, 42]. A recent meta-analyses in children with FAPDs, including five RCTs, found some beneficial effects for the use of soluble fibers, in particular psyllium, with a number needed to treat of 3. Certainty of the evidence is very low, but given the low cost, absence of serious side effects, and easy availability, soluble fiber may be considered in daily practice [9].
Low FODMAP diet
Studies in adults have shown the beneficial effect of a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) for the treatment of IBS [43]. It is hypothesized that one of the mechanisms of action involves a reduction in gas production and subsequently in luminal distention, resulting in a decrease in pain [43, 44]. A meta-analysis of adult studies on the efficacy of the low FODMAP diet showed a reduction in GI symptoms and an improved quality of life [45]. However, adherence to the low FODMAP diet is difficult, it involves high cost, and the involvement of a dietician is essential to achieve nutritional adequacy and successful treatment outcomes [46,47,48]. It is unknown when and how eliminated foods should be reintroduced, but continuing a low FODMAP diet for longer than 6 weeks is accompanied with the risk of malnutrition [49, 50]. To date, evidence-based recommendations to support the use of the low FODMAP diet in the pediatric population are lacking. Only two low-quality RCTs have been conducted, showing no efficacy, but more data from well-designed studies are needed before definitive conclusions can be drawn [51, 52]. To make the low FODMAP diet more available, new methods need to be implemented in clinical practice. The use of online apps and the widespread use of dietician-led groups may play an important role in near future [53, 54].
Gluten-free diet
In the last decade, adult studies have highlighted the potential role of gluten sensitivity as a trigger of GI symptoms in IBS [29, 30, 55]. This condition is known as non-celiac gluten sensitivity. IBS patients frequently report gluten sensitivity in the absence of a celiac disease diagnosis [30]. Future research is required to investigate the role of non-celiac gluten sensitivity in children with IBS. Currently, two pediatric IBS trials are underway, one being a double-blind placebo-controlled crossover trial evaluating the prevalence of gluten sensitivity (NCT02431585) and the other evaluating a gluten-free diet compared to a low FODMAP diet (NCT03694223).
Probiotics
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [56]. Probiotics are used to restore the altered microbiome composition, hamper the overgrowth of potentially pathogenic bacteria, and alter intestinal inflammation and permeability [57,58,59]. Since there is growing evidence for the role of the microbiome in the pathogenesis of FAPDs, probiotics may be a promising treatment option [60, 61]. A recently published Cochrane review evaluated the efficacy and safety of probiotics in children with FAPDs [12]. Meta-analyses showed moderate to high-quality evidence for the effectiveness of Lactobacillus rhamnosus GG and Lactobacillus reuteri DSM in successfully treating IBS and FAP in children [12]. There is limited evidence for the use of VSL#3.
Psychological interventions
Psychosocial interventions, such as cognitive behavioral therapy (CBT) and hypnotherapy (HT), have proven to be successful in the management of pediatric FAPDs (Table 2) [15].
Cognitive behavior therapy
Cognitive behavioral therapy (CBT) aims to alter the behaviors, cognitions, and emotions, that may contribute to IBS symptom escalation or maintenance [62,63,64]. Children and parents are taught to implement different coping and distraction strategies, and often also relaxation techniques, to decrease symptoms. CBT can be provided in various settings, such as face-to-face therapy [65,66,67], to parents via the telephone, [68] or targeted to children via the Internet [69,70,71]. A systematic review and meta-analysis in children aged 4–18 years with FAPDs included 17 studies (N = 1760) of CBT [10]. This SR found moderate certainty evidence that CBT leads to significant reduction in pain intensity and frequency scores compared with no intervention with a number needed to treat of 5. There was low certainty evidence that found that there is no difference between CBT and educational support in reducing pain intensity and frequency scores. Limitations of CBT are that there may be limited access to mental health professionals and that insurance may not cover treatment. To overcome the low availability of mental health professions, Internet-delivered and telephone-delivered CBT have shown to be effective alternatives, potentially reduce healthcare costs, and increase the availability of treatment [69,70,71,72].
Hypnotherapy
In HT, a patient is induced into a hypnotic state. During this state, a therapist guides the patient to respond to suggestions to alter its subjective experiences, perception, emotion, sensation, and thoughts or behavior [73, 74]. HT can be provided individually by a therapist [75, 76], or home-based by the use of HT-exercises on CD [77, 78]. Eight RCTs of children with IBS or FAP-NOS (6–18 years of age; N = 496) found low certainty results indicating that HT (both individually by a therapist or as self-exercise using a CD) may be an effective treatment option (number needed to treat = 5) [10]. Even in the long-term, there is a continued benefit of HT at 5-years follow-up [79, 80]. One of the disadvantages of HT is the lack of enough well-trained hypnotherapists, its time investment, and the lack of coverage by healthcare insurances. Home-based HT using standardized scripts is an attractive alternative treatment option and was originally developed to make hypnosis for children with IBS and FAP-NOS more widely available, especially in countries or areas with a low number of licensed hypnotherapists or with high costs for therapist. It has proven to be non-inferior to individual HT by a therapist at 1-year and 5-year follow-up [77, 80]. To date, online packages with ready-to-use HT exercises for at home use, together with an instruction manual and additional video material, are available for children in English, Spanish, and Dutch [81,82,83].
Yoga
Yoga practice using meditation techniques and breathing practices in combination with physical poses has been shown to improve body tone, reduce anxiety, and heighten feelings of well-being [84]. Three RCTs, including 127 children with IBS or FAP, have been performed to evaluate the effect of yoga [85,86,87]. After meta-analysis, no differences in treatment success were found between the yoga intervention and the control group [10]. Studies were of low quality since only small groups of children were included and methodological shortcomings. Therefore, there is no evidence to recommend yoga as a routine intervention in the management of pediatric FAPDs.
Other forms of complementary and alternative medicine
To date, the efficacy of complementary therapies such as acupuncture, herbal therapy, homeopathy, chiropractic therapy, or osteopathy have not been evaluated in pediatric clinical FAPD trials [10]. However, these alternative therapies are used by about 40% of children diagnosed with FAPDs [88, 89]. Potential reasons for using complementary and alternative medicine are the lack of perceived benefit of conventional therapy and its associated side effects [89]. More research in this field is clearly needed.
Other treatments
Neurostimulation
Percutaneous electrical nerve field stimulation (PENFS) to the outer ear targets specific pain areas in the central nervous system. By stimulating auricular branches of nerves that allow accessing the central nervous system, also visceral hypersensitivity can be modulated [90]. A large randomized, sham-controlled study assessed the efficacy of PENFS in the external ear in 115 children with FAPDs. Compared with the sham control group, PENFS treatment improved well-being with a significant reduction in pain and disability. Furthermore, beneficial effects were sustained at follow-up [90]. Although more evidence is needed, these data suggest that PENFS may be a good and safe non-pharmacological treatment option for pediatric FAPDs.
Fecal microbiota transplantation
Fecal microbiota transplantation targets the microbiome and may be a potential future therapeutic strategy in IBS patients. However, results in adult IBS studies have shown conflicting results and data in the pediatric population is lacking. Therefore, no valid conclusions on the efficacy of this treatment for pediatric IBS can be drawn [91, 92]. Currently, an RCT is assessing the use of fecal microbiota transplantation for refractory IBS in adolescents (NCT03074227).
Pharmacological treatment
Based on the current evidence, it is not possible to recommend any specific pharmacological treatment for the treatment of pediatric FAPDs [11]. The efficacy of several agents has been assessed for the treatment of pediatric FAPDs. Information on these studies is shown in Table 3.
Antispasmodics
Antispasmodic agents act directly on the intestinal smooth muscles to ensure relaxation, or indirectly on the nerves of the intestinal smooth muscles via receptor blockade, decreasing gastrointestinal contractions, and, consequently, alleviating abdominal pain complaints [93,94,95]. Only five RCTs have been conducted on the use of antispasmodics in children. Two studies investigated the effect of peppermint oil [96, 97], and three investigated drotaverine [98], mebeverine [99], or trimebutine [100]. A recent meta-analysis found a significant difference in treatment success between the antispasmodic and placebo groups. No difference was found in withdrawals due to adverse events [11]. However, the overall quality of the studies was very low, and results should therefore be interpreted with caution. Furthermore, these RCTs comprise small sample sizes, short-duration of therapy, and limited follow-up. More data are needed before definitive conclusions can be drawn. Currently, an RCT is investigating the effectiveness of mebeverine on abdominal pain reduction in children with IBS or FAP-NOS (Trial NL7508).
Antidepressants
Antidepressants, such as amitriptyline and citalopram, are central neuromodulators affecting the brain-gut axis. They have anticholinergic effects, decrease visceral sensitivity and GI motility, and improve mood and sleep patterns [101, 102]. A recent Cochrane review, including three RCTs, found insufficient evidence to support the use of antidepressants (amitriptyline and citalopram) in children with FAPDs [103,104,105,106]. Currently, antidepressants are commonly used in clinical practice for children who do not respond to first-line treatments [107]. However, some safety issues regarding these agents should be considered. In 2004, the Food and Drug Administration (FDA) issued boxed warnings on antidepressant drugs due to a potential increased risk of suicidality in the pediatric population [108]. In addition, the practitioner should be cautioned of the potential risk of cardiac-related side effects of tricyclic antidepressants (TCAs). Current practice advises performing an electrocardiogram to screen for prolonged QT intervals or bundle branch block before the administration of TCAs and advising families about the risks [109]. However, studies found no correlation between serious adverse cardiac events and the use of low-dose TCA in pediatric FGIDs, and side effect risks are usually reduced over time [110, 111]. More research is needed to draw firm conclusions.
Antibiotics
Rifaximin is a nonabsorbed antibiotic, which is thought to eliminate small-intestinal bacterial overgrowth. Since it is hypothesized that IBS-D patients have an abnormal microbiome, rifaximin may be a potential treatment for GI disorders [112,113,114]. In adult IBS, the use of rifaximin to treat global IBS-D symptoms has shown to be effective and safe [42, 115]. In the pediatric population, two trials were conducted on the efficacy of rifaximin. The first trial showed that, in 50 children with IBS and an abnormal lactulose breath hydrogen test, rifaximin significantly improved abdominal pain, bloating, and flatulence [116], while the other RCT, evaluating rifaximin in 75 children with FAP, found no significant difference in pain scores [117]. To date, rifaximin in pediatric IBS is not recommended. There is a long-term safety concern of rifaximin use as it may produce cross-resistant bacterial strains and interfere with the healthy microbiome in children [118].
Laxatives
A small study investigated polyethylene glycol 3350 (PEG) and tegaserod in children with IBS-C. The study found significant improvements in pain scores in the PEG + tegaserod treatment group compared with the PEG-alone group [119]. No evidence exists that PEG reduces abdominal pain in patients with IBS-C. However, PEG is commonly used as a first-line treatment for constipation since it is effective and safe. Therefore, it could be recommended to treat symptomatic constipation in IBS-C.
The relatively new therapeutic agents prucalopride (a 5-HT4 receptor agonist), and lubiprostone (prostaglandin E1 derivative) and linaclotide and plecanatide (both a guanylyl cyclase agonist) (both licensed for the management of IBS-C in adults) have shown benefits in adults with IBS-C [120,121,122]. Neither of these agents have proven efficacy in the pediatric population and are currently not approved for the treatment of IBS-C in children. Lubiprostone has been studied only in children with functional constipation and showed conflicting results [123,124,125]. Recently, the efficacy and safety of different dosages of linaclotide were evaluated in a phase 2 trial for IBS-C in children, with limited but promising results (NCT02559817). Thus, there is a clear need for large placebo-controlled RCTs evaluating the efficacy of this new compound in children with IBS-C before making any recommendations for its use.
Prokinetics
Dopamine antagonists, such as domperidone, have beneficial effects in adults with functional dyspepsia and IBS [126,127,128,129]. Only a single placebo-controlled trial assessed the efficacy of domperidone in children with FAPDs (n = 100) [130]. There was no significant difference in treatment success after 8 weeks of treatment. However, there was a significant decrease in abdominal pain intensity in the domperidone group compared with placebo. No side effects were reported. Children with FAPDs often report other symptoms, such as nausea, which is experienced by about half of the children at least twice a week [131, 132]. Therefore, domperidone treatment can be used as symptomatic treatment in children with comorbid nausea. However, caution is warranted since the use of domperidone has been associated with prolonged QT intervals and is therefore not licensed in children under the age of 12 [133, 134].
Antidiarrheal agents
Loperamide is an over-the-counter opioid receptor agonist commonly used in clinical practice to treat diarrhea [135,136,137]. However, guidelines do not recommend it as first-line treatment for adults with IBS-D since it is not effective for the most bothersome IBS symptoms, abdominal pain, and bloating [121, 135]. Although no RCTs have evaluated the efficacy of loperamide in children with IBS-D, it may still be considered for the symptomatic treatment in children with IBS-D [136].
Bile acid sequestrants
In adult and pediatric patients with IBS-D, there is some evidence that a subset of these patients has bile acid malabsorption [138,139,140]. This suggests that bile acid sequestrants could play a role in treating diarrheal symptoms in IBS. Several agents have indeed been shown to improve stool consistency in adults with IBS-D, such as cholestyramine, colestipol, and colesevelam [138, 141, 142]. To date, no well-designed studies have evaluated their efficacy in children with IBS.
Placebo
In pediatric FAPDs, the placebo response is substantial, with on average 41% of children improving on placebo [143]. Different factors are significant contributors to the placebo effect, such as the natural course of the disease, methodological bias, regression to the mean, and contextual factors. Contextual factors, including expectations and conditioning are known as the “true placebo-effect” [144,145,146]. Healthcare professionals should be mindful of the “true placebo-effect,” since this can be influenced by an active listening approach and a warm physician–patient relationship, potentially leading to positive patient expectations and thus improved treatment responses [147, 148].
It is interesting to better understand whether the placebo-effect is still present when the patient is aware of taking a placebo. A study in children has shown a beneficial effect of non-deceptive placebo in children with FAPDs [149]. A large open-label study is currently assessing the efficacy of open-label placebo in children with FGIDs (NCT02389998). Similar trials in adults with IBS have shown promising results [150, 151].
Novel treatments in adults with IBS
In the adult population, management is mostly based on the predominant symptom of the bowel dysfunction: constipation/bloating (IBS-C) or diarrhea (IBS-D) [152].
In adults with constipation-predominant IBS several treatment are in development [153]. Mizagliflozin (a SGLT1 inhibitor) reduces the uptake of sodium ions from the lumen, resulting in water retention in the lumen and loose stools. A phase 2 placebo-controlled trial in adults with IBS-C showed that mizagliflozin had significantly higher response rates than placebo and also appeared to be safe [154]. Furthermore, tenapanor (a sodium-hydrogen exchanger inhibitor) have proven to be effective and safe in phase 2 trials in adults with IBS-C [155].
Novel approaches for adults with IBS-D include opioid mediators, such as eluxadoline (a mixed opioid receptor agonist and antagonist), which has shown to be effective and safe [115, 156, 157]. However, eluxadoline has limitations in its use, since patients with a previous cholecystectomy report sphincter of Oddi spasms and pancreatitis [157]. The efficacy of eluxadoline is currently assessed in adolescents with IBS-D (NCT03339128).
Serum bovine-derive immunoglobulin (SBI) modulates junctional regulatory proteins in the gut and may therefore be a potential effective treatment [158]. Two pilot RCTs in adolescents with IBS-D have examined the effect of this drug, but have shown conflicting results [159, 160].
Ibodutant is a selective neurokinin-2 receptor antagonist and has proven to be effective and safe in phase 2 trials in adults with IBS-D [161].
Putting it all together
The heterogeneity of pediatric IBS and FAP-NOS, even within individual subtypes, makes it challenging to design a treatment algorithm to fit all children. It is known that up to 40% of children remain symptomatic despite treatment [162,163,164]. A stepwise approach, including a positive diagnostic strategy with minimal investigations, involving patients and families in shared decision-making, and an individualized approach to management, is the fundaments of IBS and FAP-NOS management. We propose a tailor-made approach for each patient, based on the family’s beliefs, published evidence when available, and the treatment of comorbid symptoms such as nausea, bloating, diarrhea, or constipation. Both non-pharmacological and pharmacological interventions should be discussed (Fig. 1). The first recommended step in the management of both IBS and FAP-NOS is validation, education, providing a positive diagnosis, and identifying stress factors. Initial treatment should include parental distraction, and simple dietary changes. When symptoms persist, especially in patients with functional disability, (online) psychological treatments could be proposed since those have proven to be successful therapies. However, while CBT or HT might be accepted by some, others might prefer pharmacological therapies or a combination of interventions. It is important to emphasize that although there is limited data to substantiate the efficacy of the combination of different interventions, those could be combined. If patients have IBS with constipation, we recommend increasing soluble fibers or laxatives, such as PEG. Diarrhea may be ameliorated with loperamide. For children with troublesome and persistent IBS-D symptoms, rifaximin and bile acid sequestrants may help. Special attention should be paid to non-abdominal pain symptoms, such as headache and chest-, back-, joint-, and extremity (arms and legs) pain [165]. These comorbid somatic symptoms are present in almost 75% of children and are associated with increased abdominal pain frequency and severity, functional disability, poor sleep, psychosocial distress, and lower health-related quality of life, potentially influencing long-term prognosis [165,166,167,168]. Additional therapy with analgesics, such as non-steroidal anti-inflammatory drugs or paracetamol, could be considered to treat these complaints. It is important to emphasize that the majority of patients can be treated with first-line management. However, various highly effective therapies (dietary and psychological interventions) are not easily available as a result of a lack of insurance coverage and also because of a lack of allied healthcare professionals. New developments include the delivery of online psychological therapies, through audiotapes, by phone, or via the Internet. Referral to a pediatric gastroenterologist experienced in pain disorders is required if first-line management fails, or if therapy to TCAs, such as amitriptyline, and PENF is considered, since these treatments are not commonly used in daily clinical practice.
A multidisciplinary approach to provide patient support is ideal, however, not always possible.
Conclusion
In conclusion, IBS and FAP-NOS are common in childhood, though no evidence-based international management guidelines are available. We suggest using a stepwise individualized approach to management, where after first-line management, both non-pharmacological and pharmacological interventions should be discussed. More high-quality intervention studies in these patient groups are necessary to guide adequate clinical management in the future.
Abbreviations
- CBT:
-
Cognitive behavior therapy
- FAP:
-
Functional abdominal pain
- FAPD:
-
Functional abdominal pain disorders
- FAP-NOS:
-
Functional abdominal pain-not otherwise specified
- FGID:
-
Functional gastrointestinal disorder
- FODMAP:
-
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols
- HT:
-
Hypnotherapy
- IBS:
-
Irritable bowel syndrome
- IBS-C :
-
Irritable bowel syndrome, predominant constipation
- IBS-D:
-
Irritable bowel syndrome, predominant-diarrhea
- IBS-M:
-
Irritable bowel syndrome mixed or alternating stool forms
- IBS-U:
-
Irritable bowel syndrome unclassified
- GI:
-
Gastrointestinal
- PEG:
-
Polyethylene glycol
- PENFS:
-
Percutaneous electrical nerve field stimulation
- TCA:
-
Tricyclic antidepressant
References
Hyams JS et al (2016) Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology 150:1456-1468e2
Korterink JJ, Diederen K, Benninga MA, Tabbers MM (2015) Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLoS ONE 10:e0126982
Rajindrajith S, Devanarayana NM (2012) Subtypes and symptomatology of irritable bowel syndrome in children and adolescents: a school-based survey using Rome III Criteria. J Neurogastroenterol Motil 18:298–304
Dhroove G, Chogle A, Saps M (2010) A million-dollar work-up for abdominal pain: is it worth it?. J Pediatr Gastroenterol Nutr 51:579–583
Youssef NN, Murphy TG, Langseder AL, Rosh JR (2006) Quality of life for children with functional abdominal pain: a comparison study of patients’ and parents’ perceptions. Pediatrics 117:54–59
Varni JW et al (2015) Health-related quality of life in pediatric patients with functional and organic gastrointestinal diseases. J Pediatr 166:85-90.e2
Drossman DA (2016) Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology 150:1262-1279e2
Higgins JPT et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:1–9
de Bruijn CMA et al (2022) Dietary interventions for functional abdominal pain disorders in children: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol null-null. https://doi.org/10.1080/17474124.2022.2055547
Gordon M, Sinopoulou V, Tabbers M et al (2022) Psychosocial interventions for the treatment of functional abdominal pain disorders in children: a systematic review and meta-analysis. JAMA Pediatr. Published online April 11, 2022. https://doi.org/10.1001/jamapediatrics.2022.0313
Rexwinkel R, de Bruijn CMA, Gordon M, Benninga MA, Tabbers MM (2021) Pharmacologic treatment in functional abdominal pain disorders in children: a systematic review. Pediatrics 147(6):e2020042101. https://doi.org/10.1542/peds.2020-042101. PMID: 34045320
Wallace C, Gordon M, Sinopoulou V, Akobeng A (2021) Probiotics for management of functional abdominal pain disorders in children. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858
Schechter NL, Coakley R, Nurko S (2021) The golden half hour in chronic pediatric pain-feedback as the first intervention. JAMA Pediatr 175:7–8
Brusaferro A, Farinelli E, Zenzeri L, Cozzali R, Esposito S (2018) The management of paediatric functional abdominal pain disorders: latest evidence. Pediatr Drugs 20:235–247
Thapar N et al (2020) Paediatric functional abdominal pain disorders. Nat Rev Dis Prim 6:89
Walker LS et al (2006) Parent attention versus distraction: impact on symptom complaints by children with and without chronic functional abdominal pain. Pain 122:43–52
Lindley KJ, Glaser D, Milla PJ (2005) Consumerism in healthcare can be detrimental to child health: lessons from children with functional abdominal pain. Arch Dis Child 90:335–337
Crushell E et al (2003) Importance of parental conceptual model of illness in severe recurrent abdominal pain. Pediatrics 112:1368–1372
Cooper TE et al (2017a) Opioids for chronic non-cancer pain in children and adolescents. Cochrane Database Syst Rev 7:CD012538
Cooper TE et al (2017b) Paracetamol (acetaminophen) for chronic non-cancer pain in children and adolescents. Cochrane Database Syst Rev 8:CD012539
Eccleston C, Cooper TE, Fisher E, Anderson B, Wilkinson NM (2017) Non-steroidal anti-inflammatory drugs (NSAIDs) for chronic non-cancer pain in children and adolescents. Cochrane Database Syst Rev 8:CD012537
Chumpitazi BP (2018) Update on Dietary Management of Childhood Functional Abdominal Pain Disorders. Gastroenterol Clin North Am 47:715–726
Chumpitazi BP, Weidler EM, Lu DY, Tsai CM, Shulman RJ (2016) Self-perceived food intolerances are common and associated with clinical severity in childhood irritable bowel syndrome. J Acad Nutr Diet 116:1458–1464
Deiteren A et al (2010) Effect of meal ingestion on ileocolonic and colonic transit in health and irritable bowel syndrome. Dig Dis Sci 55:384–391
Lea R, Whorwell PJ (2005) The role of food intolerance in irritable bowel syndrome. Gastroenterol Clin North Am 34:247–255
Camilleri M et al (2008) Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 6:772–781
van Tilburg MAL, Felix CT (2013) Diet and functional abdominal pain in children and adolescents. J Pediatr Gastroenterol Nutr 57:141–148
Turco R et al (2018) Does a low FODMAPs diet reduce symptoms of functional abdominal pain disorders?. A systematic review in adult and paediatric population, on behalf of Italian Society of Pediatrics. Ital J Pediatr 44:53
Biesiekierski JR et al (2011) Gluten Causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol 106:508–514
Barbaro MR, Cremon C, Wrona D, Fuschi D, Marasco G, Stanghellini V, Barbara G (2020) Non-celiac gluten sensitivity in the context of functional gastrointestinal disorders. Nutrients 12(12):3735. https://doi.org/10.3390/nu12123735. PMID: 33291590; PMCID: PMC7761787
Korczak R, Kamil A, Fleige L, Donovan SM, Slavin JL (2017) Dietary fiber and digestive health in children. Nutr Rev 75:241–259
Axelrod CH, Saps M (2018) The role of fiber in the treatment of functional gastrointestinal disorders in children. Nutrients 10(11):1650. https://doi.org/10.3390/nu10111650. PMID: 30400292; PMCID: PMC6267171
Paulo AZ, Amancio OMS, de Morais MB, Tabacow KMMD (2006) Low-dietary fiber intake as a risk factor for recurrent abdominal pain in children. Eur J Clin Nutr 60:823–827
Huang RC, Palmer LJ, Forbes DA (2000) Prevalence and pattern of childhood abdominal pain in an Australian general practice. J Paediatr Child Health 36:349–353
Connell AM (1978) The effects of dietary fiber on gastrointestinal motor function. Am J Clin Nutr 31:S152–S156
Romano C, Comito D, Famiani A, Calamarà S, Loddo I (2013) Partially hydrolyzed guar gum in pediatric functional abdominal pain. World J Gastroenterol 19:235–240
Anheyer D, Frawley J, Koch AK, Lauche R, Langhorst J, Dobos G, Cramer H (2017) Herbal medicines for gastrointestinal disorders in children and adolescents: a systematic review. Pediatrics 139(6):e20170062. https://doi.org/10.1542/peds.2017-0062. Epub 2017 May 4. PMID: 28562281
Shulman RJ et al (2017) Psyllium fiber reduces abdominal pain in children with irritable bowel syndrome in a randomized, double-blind trial. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 15:712-719.e4
Eswaran S, Muir J, Chey WD (2013) Fiber and functional gastrointestinal disorders. Am J Gastroenterol 108:718–727
Moayyedi P et al (2014) The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol 109:1367–1374
Nagarajan N et al (2015) The role of fiber supplementation in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 27:1002–1010
Lacy BE et al (2021) ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol 116:17–44
Staudacher HM, Whelan K (2017) The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut 66:1517–1527
Shepherd SJ, Lomer MCE, Gibson PR (2013) Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol 108:707–717
van Lanen A-S, de Bree A, Greyling A (2021) Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: a systematic review and meta-analysis. Eur J Nutr 60:3505–3522
Rej A et al (2021) The low FODMAP diet for IBS; A multicentre UK study assessing long term follow up. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver 53:1404–1411
Gibson PR, Shepherd SJ (2010) Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol 25:252–258
Alfaro-Cruz L, Heitkemper M, Chumpitazi BP, Shulman RJ (2020) Literature review: dietary intervention adherence and adherence barriers in functional gastrointestinal disorder studies. J Clin Gastroenterol 54:203–211
Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG (2014) A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 146:67-75.e5
Bellini M, Tonarelli S, Nagy AG, Pancetti A, Costa F, Ricchiuti A, de Bortoli N, Mosca M, Marchi S, Rossi A (2020) Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 12(1):148. https://doi.org/10.3390/nu12010148. PMID: 31947991; PMCID: PMC7019579
Boradyn KM, Przybyłowicz KE, Jarocka-Cyrta E (2020) Low FODMAP diet is not effective in children with functional abdominal pain: a randomized controlled trial. Ann Nutr Metab 76:334–344
Chumpitazi BP et al (2015) Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther 42:418–427
Mitchell H, Porter J, Gibson PR, Barrett J, Garg M (2019) Review article: implementation of a diet low in FODMAPs for patients with irritable bowel syndrome—directions for future research. Aliment Pharmacol Ther 49:124–139
Chen J, Gemming L, Hanning R, Allman-Farinelli M (2018) Smartphone apps and the nutrition care process: Current perspectives and future considerations. Patient Educ Couns 101:750–757
Llanos-Chea A, Fasano A (2018) Gluten and functional abdominal pain disorders in children. Nutrients 10(10):1491. https://doi.org/10.3390/nu10101491. PMID: 30322070; PMCID: PMC6212938
Hill C et al (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Quigley EMM (2008) Probiotics in functional gastrointestinal disorders: what are the facts?. Curr Opin Pharmacol 8:704–708
Martin CR, Osadchiy V, Kalani A, Mayer EA (2018) The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol 6:133–148
Cristofori F et al (2021) Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol 12:578386
Rigsbee L et al (2012) Quantitative profiling of gut microbiota of children with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 107:1740–1751
Saulnier DM et al (2011) Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 141:1782–1791
Abbott RA et al (2017) Psychosocial interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev 1:CD010971
Reed B, Buzenski J, van Tilburg MAL (2020) Implementing psychological therapies for gastrointestinal disorders in pediatrics. Expert Rev Gastroenterol Hepatol 14:1061–1067
Person H, Keefer L (2019) Brain-gut therapies for pediatric functional gastrointestinal disorders and inflammatory bowel disease. Curr Gastroenterol Rep 21:12
Abbott RA et al (2018) Recurrent abdominal pain in children: summary evidence from 3 systematic reviews of treatment effectiveness. J Pediatr Gastroenterol Nutr 67:23–33
Levy RL et al (2010) Cognitive-behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. Am J Gastroenterol 105:946–956
Levy RL et al (2013) Twelve-month follow-up of cognitive behavioral therapy for children with functional abdominal pain. JAMA Pediatr 167:178–184
Levy RL et al (2017) Brief telephone-delivered cognitive behavioral therapy targeted to parents of children with functional abdominal pain: a randomized controlled trial. Pain 158:618–628
Bonnert M et al (2017) Internet-delivered cognitive behavior therapy for adolescents with irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol 112:152–162
Nieto R, Boixadós M, Ruiz G, Hernández E, Huguet A (2019) Effects and experiences of families following a web-based psychosocial intervention for children with functional abdominal pain and their parents: a mixed-methods pilot randomized controlled trial. J Pain Res 12:3395–3412
Walker LS et al (2021) Internet-delivered cognitive behavioral therapy for youth with functional abdominal pain: a randomized clinical trial testing differential efficacy by patient subgroup. Pain
Sampaio F et al (2019) Cost-effectiveness of internet-delivered cognitive-behavioural therapy for adolescents with irritable bowel syndrome. BMJ Open 9:e023881
Green JP, Barabasz AF, Barrett D, Montgomery GH (2005) Forging ahead: the 2003 APA Division 30 definition of hypnosis. Int J Clin Exp Hypn 53:259–264
Whorwell PJ, Houghton LA, Taylor EE, Maxton DG (1992) Physiological effects of emotion: assessment via hypnosis. Lancet (London, England) 340:69–72
Vlieger AM, Menko-Frankenhuis C, Wolfkamp SCS, Tromp E, Benninga MA (2007) Hypnotherapy for children with functional abdominal pain or irritable bowel syndrome: a randomized controlled trial. Gastroenterology 133:1430–1436
Gulewitsch MD, Müller J, Hautzinger M, Schlarb AA (2013) Brief hypnotherapeutic-behavioral intervention for functional abdominal pain and irritable bowel syndrome in childhood: a randomized controlled trial. Eur J Pediatr 172:1043–1051
Rutten JMTM et al (2017) Home-based hypnotherapy self-exercises vs individual hypnotherapy with a therapist for treatment of pediatric irritable bowel syndrome, functional abdominal pain, or functional abdominal pain syndrome a randomized clinical trial. JAMA Pediatr 171:470–477
van Tilburg MAL et al (2009) Audio-recorded guided imagery treatment reduces functional abdominal pain in children: a pilot study. Pediatrics 124:e890–e897
Vlieger AM, Rutten JMTM, Govers AMAP, Frankenhuis C, Benninga MA (2012) Long-term follow-up of gut-directed hypnotherapy vs. standard care in children with functional abdominal pain or irritable bowel syndrome. Am J Gastroenterol 107:627–631
Rexwinkel R, Bovendeert JFM, Rutten JMTM, Frankenhuis C, Benninga MA, Vlieger AM (2022) Long-term follow-up of individual therapist delivered and standardized hypnotherapy recordings in pediatric irritable bowel syndrome or functional abdominal pain. JPGN (in press)
Hypnosebijbuikpijn. Available at: https://hypnosebijbuikpijn.nl/. (Accessed: 7 Nov 2021)
hypnosis4abdominalpain. Available at: https://hypnosis4abdominalpain.com/. (Accessed: 7 Nov 2021)
Hipnosis dolor abdominal. Available at: http://hipnosisdolorabdominal.com/. (Accessed: 7 Nov 2021)
Collins C (1998) Yoga: intuition, preventive medicine, and treatment. J Obstet Gynecol neonatal Nurs JOGNN 27:563–568
Evans S et al (2014) Iyengar yoga for adolescents and young adults with irritable bowel syndrome. J Pediatr Gastroenterol Nutr 59:244–253
Korterink JJ, Ockeloen LE, Hilbink M, Benninga MA, Deckers-Kocken JM (2016) Yoga therapy for abdominal pain-related functional gastrointestinal disorders in children: a randomized controlled trial. J Pediatr Gastroenterol Nutr 63:481–487
Kuttner L et al (2006) A randomized trial of yoga for adolescents with irritable bowel syndrome. Pain Res Manag 11:217–224
Day AS (2002) Use of complementary and alternative therapies and probiotic agents by children attending gastroenterology outpatient clinics. J Paediatr Child Health 38:343–346
Vlieger AM, Blink M, Tromp E, Benninga MA (2008) Use of complementary and alternative medicine by pediatric patients with functional and organic gastrointestinal diseases: results from a multicenter survey. Pediatrics 122:e446–e451
Kovacic K et al (2017) Neurostimulation for abdominal pain-related functional gastrointestinal disorders in adolescents: a randomised, double-blind, sham-controlled trial. Lancet Gastroenterol Hepatol 2:727–737
Halkjær SI et al (2018) Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut 67:2107–2115
Johnsen PH et al (2018) Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol 3:17–24
Hawthorn M et al (1988) The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment Pharmacol Ther 2:101–118
Poynard T, Regimbeau C, Benhamou Y (2001) Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 15:355–361
Annaházi A, Róka R, Rosztóczy A, Wittmann T (2014) Role of antispasmodics in the treatment of irritable bowel syndrome. World J Gastroenterol 20:6031–6043
Asgarshirazi M, Shariat M, Dalili H (2015) Comparison of the effects of pH-dependent peppermint oil and synbiotic lactol (Bacillus coagulans+ fructooligosaccharides) on childhood functional abdominal pain: a randomized placebo-controlled study. Iran Red Crescent Med J 17:0–5
Kline RM, Kline JJ, Di Palma J, Barbero GJ (2001) Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr 138:125–128
Narang M, Shah D, Akhtar H (2015) Efficacy and safety of drotaverine hydrochloride in children with recurrent abdominal pain: a randomized placebo controlled trial. Indian Pediatr 52:847–851
Pourmoghaddas Z, Saneian H, Roohafza H, Gholamrezaei A (2014) Mebeverine for pediatric functional abdominal pain: a randomized, placebo-controlled trial. Biomed Res Int 2014:191026. https://doi.org/10.1155/2014/191026. Epub 2014 Jun 25. PMID: 25089264; PMCID: PMC4095832
Karabulut GS et al (2013) The incidence of irritable bowel syndrome in children using the rome iii criteria and the effect of trimebutine treatment. J Neurogastroenterol Motil 19:90–93
Drossman DA et al (2018) Neuromodulators for functional gastrointestinal disorders (disorders of gut−brain interaction): a Rome Foundation Working Team Report. Gastroenterology 154:1140-1171.e1
Törnblom H, Drossman DA (2015) Centrally targeted pharmacotherapy for chronic abdominal pain. Neurogastroenterol Motil 27:455–467
de Bruijn CMA, Rexwinkel R, Gordon M, Benninga M, Tabbers MM (2021) Antidepressants for functional abdominal pain disorders in children and adolescents. Cochrane Database Syst Rev 2(2):CD008013. https://doi.org/10.1002/14651858.CD008013.pub3. PMID: 33560523; PMCID: PMC8094232
Roohafza H, Pourmoghaddas Z, Saneian H, Gholamrezaei A (2014) Citalopram for pediatric functional abdominal pain: a randomized, placebo-controlled trial. Neurogastroenterol Motil 26:1642–1650
Saps M et al (2009) Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology 137:1261–1269
Bahar RJ, Collins BS, Steinmetz B, Ament ME (2008) Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr 152:685–689
Rome Foundation (2020) GI genius interactive clinical decision toolkit. Rome Foundation https://romeonline.org/product/rome-iv-interactive-clinical-decisiontoolkit-logicnets
Federal Drug Administration (FDA) (2018) Suicidality in children and adolescents being treated with antidepressant medications. FDA https://www.fda.gov/drugs/postmarket-drug-safety-information-patientsand-providers/suicidality-children-and-adolescentsbeing-treated-antidepressant-medications
Li M, Ramos LG (2017) Drug-Induced QT Prolongation And Torsades de Pointes. Pharm Ther 42:473–477
Klein LJ, Chamberlain RC, Bonello K, Milazzo AS, Noel RJ (2021) Electrocardiogram before tricyclic antidepressant use: minimal impact in pediatric functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr 73:523–528
Chogle A, Saps M (2014) Electrocardiograms changes in children with functional gastrointestinal disorders on low dose amitriptyline. World J Gastroenterol 20:11321–11325
Schoenfeld P et al (2014) Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double-blind, placebo-controlled trials. Aliment Pharmacol Ther 39:1161–1168
Lembo A et al (2016) Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology 151:1113–1121
Fodor AA et al (2019) Rifaximin is associated with modest, transient decreases in multiple taxa in the gut microbiota of patients with diarrhoea-predominant irritable bowel syndrome. Gut Microbes 10:22–33
Black CJ et al (2020) Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut 69:74–82
Scarpellini E et al (2013) Rifaximin treatment for small intestinal bacterial overgrowth in children with irritable bowel syndrome. Eur Rev Med Pharmacol Sci 17:1314–1320
Collins BS, Lin HC (2011) Double-blind, placebo-controlled antibiotic treatment study of small intestinal bacterial overgrowth in children with chronic abdominal pain. J Pediatr Gastroenterol Nutr 52:382–386
Bruzzese E, Pesce M, Sarnelli G, Guarino A (2018) Pharmacokinetic drug evaluation of rifaximin for treatment of diarrhea-predominant irritable bowel syndrome. Expert Opin Drug Metab Toxicol 14:753–760
Khoshoo V, Armstead C, Landry L (2006) Effect of a laxative with and without tegaserod in adolescents with constipation predominant irritable bowel syndrome. Aliment Pharmacol Ther 23:191–196
Shah ED, Kim HM, Schoenfeld P (2018) Efficacy and tolerability of guanylate cyclase-C agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol 113:329–338
Ford AC et al (2018) American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol 113:1–18
Barish CF, Crozier RA, Griffin PH (2019) Long-term treatment with plecanatide was safe and tolerable in patients with irritable bowel syndrome with constipation. Curr Med Res Opin 35:81–85
Hyman PE, Di Lorenzo C, Prestridge LL, Youssef NN, Ueno R (2014) Lubiprostone for the treatment of functional constipation in children. J Pediatr Gastroenterol Nutr 58:283–291
Benninga MA et al (2021) Lubiprostone for pediatric functional constipation: randomized, controlled, double-blind study with long-term extension. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. https://doi.org/10.1016/j.cgh.2021.04.005
Benninga M, Hussain S, Sood M (2018) Efficacy and safety of lubiprostone in children with functional constipation: a multicenter, randomized, placebo-controlled, double-blind pivotal study. Gastroenterology 154:S559–60 (abstr)
Arts E, Anthoni H, de Roy G, D’Hollander J, Verhaegen H (1979) Domperidone in the treatment of dyspepsia: a double-blind placebo-controlled study. J Int Med Res 7:158–161
Milo R (1980) Use of the peripheral dopamine antagonist, domperidone, in the management of gastrointestinal symptoms in patients with irritable bowel syndrome. Curr Med Res Opin 6:577–584
Van de Mierop L, Rutgeerts L, Van den Langenbergh B, Staessen A (1979) Oral domperidone in chronic postprandial dyspepsia A double-blind placebo-controlled evaluation. Digestion 19:244–250
Sarin SK, Sharma P, Chawla YK, Gopinath P, Nundy S (1986) Clinical trial on the effect of domperidone on non-ulcer dyspepsia. Indian J Med Res 83:623–628
Karunanayake A, Devanarayana NM, De Silva A, Gunawardena S, Rajindrajith S (2018) Randomized controlled clinical trial on value of domperidone in functional abdominal pain in children. J Pediatr Gastroenterol Nutr 66:725–731
Kovacic K, Williams S, Li BUK, Chelimsky G, Miranda A (2013) High prevalence of nausea in children with pain-associated functional gastrointestinal disorders: are Rome criteria applicable?. J Pediatr Gastroenterol Nutr 57:311–315
Russell AC, Stone AL, Walker LS (2017) Nausea in children with functional abdominal pain predicts poor health outcomes in young adulthood. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 15:706–711
Morris AD, Chen J, Lau E, Poh J (2016) Domperidone-associated QT interval prolongation in non-oncologic pediatric patients: a review of the literature. Can J Hosp Pharm 69:224–230
European Medicines Agency (2014) Restrictions on the use of domperidone-containing medicines. Available at: https://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Domperidone_31/European_Commission_final_decision/WC500172573.pdf. Accessed 14 Aug 2021
Cangemi DJ, Lacy BE (2019) Management of irritable bowel syndrome with diarrhea: a review of nonpharmacological and pharmacological interventions. Therap Adv Gastroenterol 12:1756284819878950
Lacy BE (2016) Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med 9:7–17
Florez ID et al (2018) Comparative effectiveness and safety of interventions for acute diarrhea and gastroenteritis in children: a systematic review and network meta-analysis. PLoS ONE 13:e0207701
Vijayvargiya P et al (2018) Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 16:522–527
Saps M, Miranda A (2017) Gastrointestinal Pharmacology. Handb Exp Pharmacol 239:147–176
Beinvogl BC et al (2021) Markers of bile acid metabolism in pediatric diarrhea predominant irritable bowel syndrome and healthy controls. J Pediatr Gastroenterol Nutr 72:859–865
Camilleri M et al (2015) Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 41:438–448
Camilleri M (2015) Bile Acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver 9:332–339
Hoekman DR et al (2017) The placebo response in pediatric abdominal pain-related functional gastrointestinal disorders: a systematic review and meta-analysis. J Pediatr 182:155-163.e7
Kirsch I (2013) The placebo effect revisited: lessons learned to date. Complement Ther Med 21:102–104
Elsenbruch S, Enck P (2015) Placebo effects and their determinants in gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 12:472–485
Benninga MA, Mayer EA (2009) The power of placebo in pediatric functional gastrointestinal disease. Gastroenterology 137:1207–1210
Kelley JM et al (2009) Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom Med 71:789–797
Kaptchuk TJ et al (2008) Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 336:999–1003
Nurko S, Saps M, Kossowsky J, Zion SR, Di Lorenzo C, Vaz K, Hawthorne K, Wu R, Ciciora S, Rosen JM, Kaptchuk TJ, Kelley JM (2022) Effect of open-label placebo on children and adolescents with functional abdominal pain or irritable bowel syndrome: a randomized clinical trial. JAMA Pediatr 176(4):349–356. https://doi.org/10.1001/jamapediatrics.2021.5750. Erratum in: https://doi.org/10.1001/jamapediatrics.2022.0359. PMID: 35099543; PMCID: PMC8804974
Kaptchuk TJ et al (2010) Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS ONE 5:e15591
Lembo A et al (2021) Open-label placebo vs double-blind placebo for irritable bowel syndrome: a randomized clinical trial. Pain 162:2428–2435
Camilleri M (2021) Diagnosis and treatment of irritable bowel syndrome: a review. JAMA - J Am Med Assoc 325:865–877
Ford AC, Sperber AD, Corsetti M, Camilleri M (2020) Irritable bowel syndrome. Lancet 396:1675–1688
Fukudo S et al (2018) Safety and efficacy of the sodium-glucose cotransporter 1 inhibitor mizagliflozin for functional constipation: a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Gastroenterol Hepatol 3:603–613
Chey WD, Lembo AJ, Rosenbaum DP (2017) Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol 112:763–774
Lembo AJ et al (2016) Eluxadoline for Irritable bowel syndrome with diarrhea. N Engl J Med 374:242–253
Fragkos KC (2017) Spotlight on eluxadoline for the treatment of patients with irritable bowel syndrome with diarrhea. Clin Exp Gastroenterol 10:229–240
Sinagra E et al (2017) New therapeutic perspectives in irritable bowel syndrome: targeting low-grade inflammation, immuno-neuroendocrine axis, motility, secretion and beyond. World J Gastroenterol 23:6593–6627
Rana A, Fernandez M, Wang Z, Hyams J (2017) Safety, tolerability, and efficacy of serum-derived bovine immunoglobulin in children with diarrhea-predominant irritable bowel syndrome. Gastroenterology 152:S652
Arrouk R, Herdes RE, Karpinski AC, Hyman PE (2018) Serum-derived bovine immunoglobulin for children with diarrhea-predominant irritable bowel syndrome. Pediatr Heal Med Ther 9:129–133
Tack J et al (2017) The neurokinin-2 receptor antagonist ibodutant improves overall symptoms, abdominal pain and stool pattern in female patients in a phase II study of diarrhoea-predominant IBS. Gut 66:1403–1413
Gieteling MJ, Bierma-Zeinstra SMA, Passchier J, Berger MY (2008) Prognosis of chronic or recurrent abdominal pain in children. J Pediatr Gastroenterol Nutr 47:316–326
Gieteling MJ, Bierma-Zeinstra SM, Lisman-van Leeuwen Y, Passchier J, Berger MY (2011) Prognostic factors for persistence of chronic abdominal pain in children. J Pediatr Gastroenterol Nutr 52:154–161
Horst S et al (2014) Predicting persistence of functional abdominal pain from childhood into young adulthood. Clin Gastroenterol Hepatol 12:2026–2032
Chumpitazi BP et al (2021) Multisite pain is highly prevalent in children with functional abdominal pain disorders and is associated with increased morbidity. J Pediatr 236:131–136
Rabbitts JA, Holley AL, Groenewald CB, Palermo TM (2016) Association Between widespread pain scores and functional impairment and health-related quality of life in clinical samples of children. J pain 17:678–684
Skrove M, Romundstad P, Indredavik MS (2015) Chronic multisite pain in adolescent girls and boys with emotional and behavioral problems: the Young-HUNT study. Eur Child Adolesc Psychiatry 24:503–515
Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD (2016) Overlapping chronic pain conditions: implications for diagnosis and classification. J pain 17:T93–T107
Author information
Authors and Affiliations
Contributions
Ms. Rexwinkel conceptualized and designed the study, reviewed the literature, drafted the initial manuscript, and approved the final manuscript as submitted. Dr. Vlieger, Prof. Dr. Saps and Dr. Tabbers reviewed and revised the manuscript, and approved the final manuscript as submitted. Prof. Dr. Benninga critically reviewed and revised the manuscript for important intellectual content, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Ethics approval
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rexwinkel, R., Vlieger, A.M., Saps, M. et al. A therapeutic guide on pediatric irritable bowel syndrome and functional abdominal pain-not otherwise specified. Eur J Pediatr 181, 2603–2617 (2022). https://doi.org/10.1007/s00431-022-04459-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04459-y