Abstract
Purpose
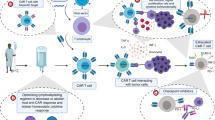
A barrier to widespread adoption of chimeric antigen receptor (CAR) T-cell therapy is toxicity. To address this, we recently developed a novel antibody-T-cell receptor (AbTCR) platform (trademarked as ARTEMIS®) which was designed to leverage natural immune receptor signaling and regulation. The AbTCR platform includes a gamma/delta (γδ) TCR-based AbTCR construct and a separate co-stimulatory molecule, both engineered to be tumor-specific. Here, we aim to assess the safety and preliminary efficacy of a CD19-directed AbTCR T-cell therapy.
Methods
We generated ET019003 T cells, which are autologous CD19-directed AbTCR T cells. We then conducted an early phase I study to evaluate the safety and preliminary efficacy of ET019003 T cells for the treatment of CD19-positive relapsed/refractory (r/r) B-cell lymphoma.
Results
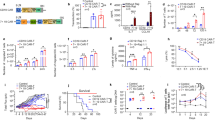
Sixteen patients enrolled in this study and 12 patients were treated. Of the 12 patients treated, 6 patients (50%) achieved a complete response (CR), and 4 (33%) achieved a partial response (PR) (best objective response rate [ORR] of 83%). CRs were durable, including 2 patients with ongoing CRs for 22.7 months and 23.2 months. ET019003 was well-tolerated with an attractive safety profile. No patients experienced severe (grade ≥ 3) cytokine release syndrome (CRS) and only 1 patient experienced immune effector cell-associated neurotoxicity syndrome (ICANS) of any grade. Significant elevations of cytokine levels were not seen, even in patients with marked expansion of ET019003 T cells.
Conclusion
This study provides initial clinical validation of the AbTCR platform as a novel cancer treatment with the potential to provide durable clinical benefit with low toxicity.
Trial registration
NCT03642496; Date of registration: August 22, 2018.



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
References
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, Sehgal A, Solomon SR, Ghosh N, Albertson TM, Garcia J, Kostic A, Mallaney M, Ogasawara K, Newhall K, Kim Y, Li D, Siddiqi T (2020) Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396(10254):839–852. https://doi.org/10.1016/s0140-6736(20)31366-0
Barrett DM, Singh N, Porter DL, Grupp SA, June CH (2014) Chimeric antigen receptor therapy for cancer. Annu Rev Med 65:333–347. https://doi.org/10.1146/annurev-med-060512-150254
Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, Quintás-Cardama A, Larson SM, Sadelain M (2007) Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res : off J Am Assoc Cancer Res 13(18 Pt 1):5426–5435. https://doi.org/10.1158/1078-0432.ccr-07-0674
Brudno JN, Lam N, Vanasse D, Shen YW, Rose JJ, Rossi J, Xue A, Bot A, Scholler N, Mikkilineni L, Roschewski M, Dean R, Cachau R, Youkharibache P, Patel R, Hansen B, Stroncek DF, Rosenberg SA, Gress RE, Kochenderfer JN (2020) Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat Med 26(2):270–280. https://doi.org/10.1038/s41591-019-0737-3
Carroll J (2021) Carl June’s Tmunity encounters a lethal roadblock as 2 patient deaths derail lead trial, raise red flag forcing a rethink of CAR-T for solid tumors. Endpoint News. https://endpts.com/exclusive-carl-junes-tmunity-encounters-a-lethal-roadblock-as-2-patient-deaths-derail-lead-trial-raise-red-flag-forcing-a-rethink-of-car-t-for-solid-tumors/. Accessed June 25, 2021
Chen L, Flies DB (2013) Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13(4):227–242. https://doi.org/10.1038/nri3405
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3068. https://doi.org/10.1200/jco.2013.54.8800
Gauthier J, Turtle CJ (2018) Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr Res Transl Med 66(2):50–52. https://doi.org/10.1016/j.retram.2018.03.003
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368(16):1509–1518. https://doi.org/10.1056/NEJMoa1215134
Jaglowski S, Hu Z-H, Zhang Y, Kamdar M, Ghosh M, Lulla P, Sasine J, Perales M-A, Hematti P, Nikiforow S, Steinert P, Jeschke M, Yi L, Chawla R, Pacaud L, Horowitz MM, Bleickardt E, Pasquini MC (2019) Tisagenlecleucel chimeric antigen receptor (CAR) T-cell therapy for adults with diffuse large B-cell lymphoma (DLBCL): real world experience from the center for international blood and marrow transplant research (CIBMTR) cellular therapy (CT) registry. Blood 134(Supplement_1):766. https://doi.org/10.1182/blood-2019-130983
Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, Xue A, Goff SL, Yang JC, Sherry RM, Klebanoff CA, Kammula US, Sherman M, Perez A, Yuan CM, Feldman T, Friedberg JW, Roschewski MJ, Feldman SA, McIntyre L, Toomey MA, Rosenberg SA (2017) Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol 35(16):1803–1813. https://doi.org/10.1200/jco.2016.71.3024
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, Go WY, Eldjerou L, Gardner RA, Frey N, Curran KJ, Peggs K, Pasquini M, DiPersio JF, van den Brink MRM, Komanduri KV, Grupp SA, Neelapu SS (2019) ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant : J Am Soc Blood Marrow Transplant 25(4):625–638. https://doi.org/10.1016/j.bbmt.2018.12.758
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, Deol A, Reagan PM, Stiff P, Flinn IW, Farooq U, Goy A, McSweeney PA, Munoz J, Siddiqi T, Chavez JC, Herrera AF, Bartlett NL, Wiezorek JS, Navale L, Xue A, Jiang Y, Bot A, Rossi JM, Kim JJ, Go WY, Neelapu SS (2019) Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 20(1):31–42. https://doi.org/10.1016/s1470-2045(18)30864-7
Maude SL, Barrett D, Teachey DT, Grupp SA (2014) Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 20(2):119–122. https://doi.org/10.1097/ppo.0000000000000035
Maude SL, Teachey DT, Porter DL, Grupp SA (2015) CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 125(26):4017–4023. https://doi.org/10.1182/blood-2014-12-580068
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA (2018) Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378(5):439–448. https://doi.org/10.1056/NEJMoa1709866
McCarten KM, Nadel HR, Shulkin BL, Cho SY (2019) Imaging for diagnosis, staging and response assessment of Hodgkin lymphoma and non-Hodgkin lymphoma. Pediatr Radiol 49(11):1545–1564. https://doi.org/10.1007/s00247-019-04529-8
Munshi NC, Anderson LD Jr, Shah N, Madduri D, Berdeja J, Lonial S, Raje N, Lin Y, Siegel D, Oriol A, Moreau P, Yakoub-Agha I, Delforge M, Cavo M, Einsele H, Goldschmidt H, Weisel K, Rambaldi A, Reece D, Petrocca F, Massaro M, Connarn JN, Kaiser S, Patel P, Huang L, Campbell TB, Hege K, San-Miguel J (2021) Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 384(8):705–716. https://doi.org/10.1056/NEJMoa2024850
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY (2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377(26):2531–2544. https://doi.org/10.1056/NEJMoa1707447
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M (2018) Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 378(5):449–459. https://doi.org/10.1056/NEJMoa1709919
Pasquini MC, Hu Z-H, Curran K, Laetsch T, Locke F, Rouce R, Pulsipher MA, Phillips CL, Keating A, Frigault MJ, Salzberg D, Jaglowski S, Sasine JP, Rosenthal J, Ghosh M, Landsburg D, Margossian S, Martin PL, Kamdar MK, Hematti P, Nikiforow S, Turtle C, Perales M-A, Steinert P, Horowitz MM, Moskop A, Pacaud L, Yi L, Chawla R, Bleickardt E, Grupp S (2020) Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv 4(21):5414–5424. https://doi.org/10.1182/bloodadvances.2020003092
Pasquini MC, Locke FL, Herrera AF, Siddiqi T, Ghobadi A, Komanduri KV, Hu Z-H, Dong H, Hematti P, Nikiforow S, Steinert P, Purdum A, Horowitz MM, Hooper M, Kawashima J, Jacobson CA (2019) Post-marketing use outcomes of an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, axicabtagene ciloleucel (Axi-Cel), for the treatment of large B cell lymphoma (LBCL) in the United States (US). Blood 134(1):764. https://doi.org/10.1182/blood-2019-124750
Pennisi M, Jain T, Santomasso BD, Mead E, Wudhikarn K, Silverberg ML, Batlevi Y, Shouval R, Devlin SM, Batlevi C, Brentjens RJ, Dahi PB, Diamonte C, Giralt S, Halton EF, Maloy M, Palomba ML, Sanchez-Escamilla M, Sauter CS, Scordo M, Shah G, Park JH, Perales MA (2020) Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv 4(4):676–686. https://doi.org/10.1182/bloodadvances.2019000952
Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ, June CH (2015) Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 7(303):303ra139. https://doi.org/10.1126/scitranslmed.aac5415
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A, Lam LP, Morgan RA, Friedman K, Massaro M, Wang J, Russotti G, Yang Z, Campbell T, Hege K, Petrocca F, Quigley MT, Munshi N, Kochenderfer JN (2019) Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med 380(18):1726–1737. https://doi.org/10.1056/NEJMoa1817226
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, Melenhorst JJ, Porter DL, June CH (2017) Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 377(26):2545–2554. https://doi.org/10.1056/NEJMoa1708566
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak Ö, Salles G, Maziarz RT (2019) Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380(1):45–56. https://doi.org/10.1056/NEJMoa1804980
Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, Pequignot E, Gonzalez VE, Chen F, Finklestein J, Barrett DM, Weiss SL, Fitzgerald JC, Berg RA, Aplenc R, Callahan C, Rheingold SR, Zheng Z, Rose-John S, White JC, Nazimuddin F, Wertheim G, Levine BL, June CH, Porter DL, Grupp SA (2016) Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 6(6):664–679. https://doi.org/10.1158/2159-8290.cd-16-0040
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG (2016a) CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Investig 126(6):2123–2138. https://doi.org/10.1172/jci85309
Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, Soma L, Wood B, Li D, Heimfeld S, Riddell SR, Maloney DG (2016b) Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 8(355):355ra116. https://doi.org/10.1126/scitranslmed.aaf8621
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, McSweeney PA, Miklos DB, Pagel JM, Kersten MJ, Milpied N, Fung H, Topp MS, Houot R, Beitinjaneh A, Peng W, Zheng L, Rossi JM, Jain RK, Rao AV, Reagan PM (2020) KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 382(14):1331–1342. https://doi.org/10.1056/NEJMoa1914347
Xu Y, Yang Z, Horan LH, Zhang P, Liu L, Zimdahl B, Green S, Lu J, Morales JF, Barrett DM, Grupp SA, Chan VW, Liu H, Liu C (2018) A novel antibody-TCR (AbTCR) platform combines fab-based antigen recognition with gamma/delta-TCR signaling to facilitate T-cell cytotoxicity with low cytokine release. Cell Discov 4(1):62. https://doi.org/10.1038/s41421-018-0066-6
Ying Z, Huang XF, Xiang X, Liu Y, Kang X, Song Y, Guo X, Liu H, Ding N, Zhang T, Duan P, Lin Y, Zheng W, Wang X, Lin N, Tu M, Xie Y, Zhang C, Liu W, Deng L, Gao S, Ping L, Wang X, Zhou N, Zhang J, Wang Y, Lin S, Mamuti M, Yu X, Fang L, Wang S, Song H, Wang G, Jones L, Zhu J, Chen SY (2019) A safe and potent anti-CD19 CAR T cell therapy. Nat Med 25(6):947–953. https://doi.org/10.1038/s41591-019-0421-7
Acknowledgements
The authors thank Jing Li, Xin Liu, Mengchang Wang, Jieying Xi, and Haitao Zhang for their involvement in the conduct of the study; Hong Liu for assistance with clinical protocol design; Joy Lin for help with data organization and manuscript preparation; and Vivien Chan, Sal Fuerstenberg, Mei Hong, Michael Kavanaugh, Nicole Nuñez, and Pei Wang for critical review of the manuscript.
Funding
This study was sponsored by The First Affiliated Hospital of Xi’an Jiaotong University in collaboration with Eureka Therapeutics, Inc. This study was supported by National Key R \(\&\) D Program of China (No. 2019YFC1316204), Key Research and Development Program of Shaanxi (No. 2018ZDCXL-SF-01–02-01, 2021ZDLSF02-17 and 2021SF-302), and National Scientific and Technological Major Special Project for Significant Creation of New Drugs (No. 2020ZX09201020).
Author information
Authors and Affiliations
Contributions
PH, MZ, HL, and CL conceived and designed the study; PH oversaw the conduct of the study; HL, JW, ML, HL, LC, and HW were involved in the conduct of the study; HL, BZ, and FT analyzed and interpreted clinical data; BZ analyzed and interpreted correlative data and performed statistical analysis; QC oversaw drug product manufacturing, Quality Control, and correlative studies; FT collected correlative data; FT and DY collected drug product data; FN oversaw the Good Manufacturing Practice laboratory and drug product manufacturing; DY analyzed drug product data and established Quality Control testing for drug product release; SAG assisted with the interpretation of clinical and correlative data; BZ wrote the manuscript; and all authors critically reviewed the manuscript and approved the content.
Corresponding author
Ethics declarations
Competing interests
BZ, QC, FT, FN, DY, and CL are employed at Eureka Therapeutics, Inc., (Eureka) and have equity and/or stock options in Eureka. CL serves on advisory boards for the following companies: Eureka and JW Therapeutics. SAG has received support from Novartis, Servier, and Kite and serves as a consultant, member of the scientific advisory board or study steering committee for Novartis, Cellectis, Adaptimmune, Eureka, TCR2, Juno, GlaxoSmithKline, Vertex, Cure Genetics, Humanigen, and Roche. The remaining authors declare no competing financial interests.
Ethical approval
This study was approved by the Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University in China. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Consent to participate
All participants in the study provided written informed consent.
Consent to publish
Patient consent for publication is not required. Patients consented to participate in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, P., Liu, H., Zimdahl, B. et al. A novel antibody-TCR (AbTCR) T-cell therapy is safe and effective against CD19-positive relapsed/refractory B-cell lymphoma. J Cancer Res Clin Oncol 149, 2757–2769 (2023). https://doi.org/10.1007/s00432-022-04132-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04132-9