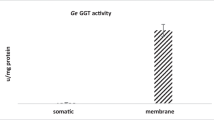
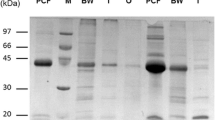
Abstract
Glucose 6-phosphate dehydrogenase (EC 1.1.1.49) was purified to homogeneity from the soluble fraction of larval Taenia crassiceps (Eucestoda: Cyclophyllidea) by a three-step protocol. Specific activity of the pure enzyme was 33.8 ± 2.1 U mg−1 at 25°C and pH 7.8 with d-glucose 6-phosphate and NADP+ as substrates. The activity increases to 67.6 ± 3.9 U mg−1 at 39°C, a more physiological temperature in the intermediary host. Enzyme activity was maximal between pH 6.7 and 7.8. K m values were 14 ± 1.7 μM and 1.3 ± 0.4 μM for glucose 6-phosphate and NADP+, respectively. The enzyme showed absolute specificity for its sugar substrate. NAD+ was also a substrate but with a low catalytic efficiency (207 M−1 s−1). No essential requirement for Mg++ or Ca++ was observed. Relative molecular mass of the native enzyme was 134,000 ± 17,200, while a value of 61,000 ± 1,700 was obtained for the enzyme subunit. Thus, glucose 6-phosphate dehydrogenase from T. crassiceps exists as a dimeric protein. The enzyme’s isoelectric point was 4.5. The enzyme’s activity dependence on temperature was complex, resulting in a biphasic Arrhenius plot. Activation energies of 9.91 ± 0.51 and 7.94 ± 0.45 kcal mol−1 were obtained. Initial velocity patterns complemented with inhibition studies by product and substrate’s analogues support a random bi bi sequential mechanism in rapid equilibrium. The low K i value of 1.95 μM found for NADPH suggests a potential regulatory role for this nucleotide.




Similar content being viewed by others
References
Agosin M, Aravena L (1960) Studies on the metabolism of Echinococcus granulosus. IV. Enzymes of the pentose phosphate pathway. Exp Parasitol 10:23–38
Agosin M, Repetto Y (1961) Studies on the metabolism of Echinococcus granulosus. VI. Pathways of glucose 14C metabolism in E. granulosus scolices. Biologica 23:33–38
Berkelmen T, Stenstedt T (1998) 2-D electrophoresis using immobilized pH gradients. Principles and methods. Amersham Pharmacia Biotech, UK
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
del Arenal IP, Rubio ME, Ramírez J, Rendón JL, Escamilla JE (2005) Cyanide-resistant respiration in Taenia crassiceps metacestode (cysticerci) is explained by the H2O2-producing side-reaction of respiratory complex I with O2. Parasitol Int 54:185–193
Freeman RS (1962) Studies on the biology of Taenia crassiceps (Zeder, 1800) Rudolphi, 1810 (Cestoda). Can J Zool 40:969–990
Glaser L, Brown DH (1955) Purification and properties of glucose 6-phosphate dehydrogenase. J Biol Chem 216:67–79
Hagel L (1998) Gel filtration. In: Janson JC, Rydén L (eds) Protein purification. Principles, high resolution methods, and applications. Wiley-VCH, New York, pp 79–143
Horecker BL, Smyrniotis PZ (1953) Reversibility of glucose-6-phosphate oxidation. Biochim Biophys Acta 12:98–102
Kanji MI, Toews ML, Carper WR (1976) A kinetic study of glucose 6-phosphate dehydrogenase. J Biol Chem 251:2258–2262
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Larralde C, Sciutto E, Grun J, Díaz ML, Govezensky T, Montoya RM (1989) Biological determinants of host–parasite relationship in mouse cysticercosis caused by Taenia crassiceps: influence of sex, major histocompatibility complex and vaccination. In: Cañedo LE, Todd LE, Packer L, Jaz J (eds) Cell function and disease. Plenum, New York, pp 325–352
Levy HR (1979) Glucose 6-phosphate dehydrogenases. Adv Enzymol Relat Areas Mol Biol 48:97–192
McManus DP, Smyth JD (1982) Intermediary carbohydrate metabolism in protoscoleces of Echinococcus granulosus (horse and sheep strains) and E. multilocularis. Parasitology 84:351–366
Olive C, Levy HR (1971) Glucose 6-phosphate dehydrogenase from Leuconostoc mesenteroides. Physical studies. J Biol Chem 246:2043–2046
Özer N, Aksoy Y, Ögüs IH (2001) Kinetic properties of human placental glucose 6-phosphate dehydrogenase. Int J Biochem Cell Biol 33:221–226
Özer N, Bilgi C, Ögüs IH (2002) Dog liver glucose-6-phosphate dehydrogenase: purification and kinetic properties. Int J Biochem Cell Biol 34:253–262
Puterman ML, Hrboticky N, Innis SM (1988) Nonlinear estimation of parameters in biphasic Arrhenius plots. Anal Biochem 170:409–420
Rendón JL, del Arenal IP, Guevara-Flores A, Uribe A, Mendoza-Hernández G (2004) Purification, characterization and kinetic properties of the multifunctional thioredoxin-glutathione reductase from Taenia crassiceps metacestode (cysticerci). Mol Biochem Parasitol 133:61–69
Segel IH (1975) Enzyme kinetics. Behavior and analysis of rapid equilibrium and steady state enzyme systems. Wiley-Interscience, New York
Shreve DS, Levy HR (1980) Kinetic mechanism of glucose 6-phosphate dehydrogenase from the lactating rat mammary gland. Implications for regulation. J Biol Chem 255:2670–2677
Smith TM, Brown JN (1977) Schistosoma mansoni and Schistosoma japonicum: comparison of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase activities in adults. Comp Biochem Physiol B 56:351–352
Ulusu NN, Kus MS, Acan NL, Tezcan EF (1999) A rapid method for the purification of glucose 6-phosphate dehydrogenase from bovine lens. Int J Biochem Cell Biol 31:787–796
Wang XT, Au SWN, Lam VMS, Engel PC (2002) Recombinant human glucose 6-phosphate dehydrogenase. Evidence for a rapid-equilibrium random-order mechanism. Eur J Biochem 269:3417–3424
Wood T (1986) Physiological functions of the pentose phosphate pathway. Cell Biochem Funct 4:241–247
Zenka J, Prokopic J (1987) Malic enzyme, malate dehydrogenase, fumarate reductase and succinate dehydrogenase in the larvae of Taenia crassiceps (Zeder 1800). Folia Parasitol 34:131–136
Acknowledgments
This work was supported by research grants IN236002-3 and IN224506-2 from Dirección General de Asuntos del Personal Académico (DGAPA), UNAM. We are also grateful to Miss Josefina Bolado for the corrections made in the language of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rendón, J.L., del Arenal, I.P., Guevara-Flores, A. et al. Glucose 6-phosphate dehydrogenase from larval Taenia crassiceps (cysticerci): purification and properties. Parasitol Res 102, 1351–1357 (2008). https://doi.org/10.1007/s00436-008-0917-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-0917-4