Abstract
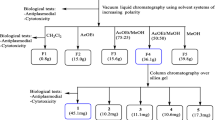
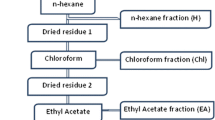
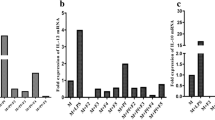
Malaria is an overwhelming impact in the poorest countries in the world due to their prevalence, virulence and drug resistance ability. Currently, there is inadequate armoury of drugs for the treatment of malaria. This underscores the continuing need for the discovery and development of new effective and safe antimalarial drugs. To evaluate the in vitro and in vivo antimalarial activity of the leaf ethyl acetate extract of Murraya koenigii, bioassay-guided chromatographic fractionation was employed for the isolation and purification of antimalarial compounds. The in vitro antimalarial activity was assayed by the erythrocytic stages of chloroquine-sensitive strain of Plasmodium falciparum (3D7) in culture using the fluorescence-based SYBR Green I assay. The in vivo assay was done by administering mice infected with Plasmodium berghei (NK65) four consecutive daily doses of the extracts through oral route following Peter’s 4-day curative standard test. The percentage suppression of parasitaemia was calculated for each dose level by comparing the parasitaemia in untreated control with those of treated mice. Cytotoxicity was determined against HeLa cells using MTT assay. Histopathology was studied in kidney, liver and spleen of isolated compound-treated Swiss albino mice. The leaf crude ethyl acetate extract of M. koenigii showed good in vitro antiplasmodial activity against P. falciparum. The in vivo test of the leaf crude ethyl acetate extract (600 mg/kg) showed reduced malaria parasitaemia by 86.6 % against P. berghei in mice. Bioassay-guided fractionation of the leaf ethyl acetate extract of M. koenigii led to the isolation of two purified fractions C3B2 (2.84 g) and C3B4 (1.97 g). The purified fractions C3B2 and C3B4 were found to be active with IC50 values of 10.5 ± 0.8 and 8.25 ± 0.2 μg/mL against P. falciparum, and in vivo activity significantly reduced parasitaemia by 82.6 and 88.2 % at 100 mg/kg/body weight on day 4 against P. berghei, respectively. The isolated fractions C3B2 and C3B4 were monitored by thin-layer chromatography until a single spot was obtained with R f values of 0.36 and 0.52, respectively. The pure compounds obtained in the present investigation were subjected to UV–visible spectroscopy, Fourier transformer infrared spectroscopy, 1D and 2D 1H-Nuclear magnetic resonance (NMR), 13C NMR, DEPT, COSY and Mass spectral analysis. Based on the spectral analysis, it is concluded that the isolated compounds were myristic acid (C3B2) and β-caryophyllene (C3B4). The cytotoxic effect of myristic acid and β-caryophyllene showed the TC50 values of >100 and 80.5 μg/mL, respectively against HeLa cell line. The histopathology study showed that protection against nephrotoxicity of kidney, hepatic damage of liver and splenocytes protection in spleen was achieved with the highest dose tested at 100 mg/kg/body weight. The present study provides evidence of antiplasmodial compounds from M. koenigii and is reported for the first time.





Similar content being viewed by others
References
Adebajo AC, Olayiwola G, Verspohl JE, Iwalewd EO, Omisore NOA, Bergenthal D (2004) Evaluation of the ethnomedical claims of Murraya koenigii. Pharm Biol 42:610–620
Adewoye EO, Salami AT, Taiwo VO (2010) Anti-plasmodial and toxicological effects of methanolic bark extract of Chrysophyllum albidum in albino mice. J Physiol Pathophysiol 1(1):1–9
Agarwal HK, Chhikara BS, Bhavaraju S, Mandal D, Doncel GF, Parang K (2013) Emtricitabine prodrugs with improved anti-HIV activity and cellular uptake. Mol Pharm 10(2):467–476
Alma MH, Mavi A, Yildirim A, Digrak M, Hirata T (2003) Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. growing in Turkey. Biol Pharm Bull 26:1725–1729
Arulselvan P, Subramanian SP (2007) Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultrastructural changes of pancreatic b-cell in experimental diabetes. Chem Biol Interact 165:155–164
Bagavan A, Rahuman AA, Kamaraj C, Geetha K (2008) Larvicidal activity of saponin from Achyranthes aspera against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 103(1):223–229
Bagavan A, Rahuman AA, Kamaraj C, Kaushik NK, Mohanakrishnan D, Sahal D (2011a) Antiplasmodial activity of botanical extracts against Plasmodium falciparum. Parasitol Res 108:1099–1109
Bagavan A, Rahuman AA, Kaushik NK, Sahal D (2011b) In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitol Res 108:15–22
Bero J, Quetin-Leclercq J (2011) Natural products and tropical diseases. Planta Med 77:571
Bhat GP, Surolia N (2001) In vitro antimalarial activity of three plants used in the traditional medicine of India. Am J Trop Med Hyg 65(4):304–308
Carrara IV, Zwang J, Asley AE, Price RA, Stepniewka K, Barends M, Brock-man A, Anderson T, McGready R, Phaiphun L, Proux S, Vugt M, van Hutagalung R, Lwin MK, PyaePhyo A, Preechapornkul P, Imwong M, Pukrittayakamee S, Singhasivanon P, White NJ, Nosten F (2009) Changes in the treatment response to artesunate–mefloquine on the north western border of Thailand during 13 years of continuous deployment. PLoS ONE 4:2
Chowdhury JU, Bhuiyan MNI, Yusuf M (2008) Chemical composition of the essential oils of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Bangladesh J Pharmacol 3:59–63
Cornwell PA, Barry BW (1994) Sesquiterpene components of volatile oils as skin penetration enhancers for the hydrophilic permeant 5-fluorouracil. J Pharm Pharmacol 46:261–269
David AF, Philip JR, Simon RC, Reto B, Solomon N (2004) Antimalarial drug discovery: efficiency models for compound screening. Nat Rev 3:509–520
Dondorp AM, Yeung S, Ngguonn C, Day NP, Socheat D, VonSeidlein L (2010) Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol 8:272–280
Doria GA, Silva WJ, Carvalho GA, Alves PB, Cavalcanti SC (2010) A study of the larvicidal activity of two Croton species from northeastern Brazil against Aedes aegypti. Pharm Biol 48(6):615–620
Dorin D, Le Roch K, Sallicandro P, Alano P, Parzy D, Poullet P, Meijer L, Doerig C (2001) Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum. Biochemical properties and possible involvement in MAPK regulation. Eur J Biochem 268:2600–2608
Ene AC, Atawodi SE, Ameh DA, Kwanshie HO, Agomo PU (2008) Experimental induction of chloroquine resistance in Plasmodium berghei NK 65. Trends Med Res 3(1):16–23
George BO, Osioma E, Okpoghono J, Aina OO (2011) Changes in liver and serum transaminases and alkaline phosphatase enzyme activities in Plasmodium berghei infected mice treated with aqueous extract of Aframomum sceptrum. Afr J Biochem Res 5(9):277–281
Ginsburg H, Deharo E (2011) A call for using natural compounds in the development of new antimalarial treatment an introduction. Malar J 10(Suppl 1):S1
Greenwood BM, Bojang K, Whitty CJM, Targett GAT (2005) Malaria. Lancet 365:1487–1498
Gupta P, Vasudeva N (2010) In vitro antiplasmodial and antimicrobial potential of Tagetes erecta roots. Pharm Biol 48(11):1218–1223
Gurib-Fakim A (2006) Medicinal plants: tradition of yesterday and drugs of tomorrow. Mol Asp Med 27:1–93
Iwalewa EO, Oguntoye L, Rai PP, Iyaniwura TT (1997) In vivo and in vitro antimalarial activity of two crude extracts of Cassia occidentalis leaf. Niger J Pharm Sci 5:23–28
Kamaraj C, Kaushik NK, Mohanakrishnan D, Elango G, Bagavan A, Zahir AA, Rahuman AA, Sahal D (2012a) Antiplasmodial potential of medicinal plant extracts from Malaiyur and Javadhu hills of South India. Parasitol Res 111(2):703–715
Kamaraj C, Kaushik NK, Rahuman AA, Mohanakrishnan D, Bagavan A, Elango G, Zahir AA, Santhoshkumar T, Marimuthu S, Jayaseelan C, Kirthi AV, Rajakumar G, Velayutham K, Sahal D (2012b) Antimalarial activities of medicinal plants traditionally used in the villages of Dharmapuri regions of South India. J Ethnopharmacol 141(3):796–802
Kaushik NK, Bagavan A, Rahuman AA, Mohanakrishnan D, Kamaraj C, Elango G, Zahir AA, Sahal D (2013) Antiplasmodial potential of selected medicinal plants from eastern Ghats of South India. Exp Parasitol 134(1):26–32
Keluskar P, Ingle S (2012) Ethnopharmacology guided screening of traditional Indian herbs for selective inhibition of Plasmodium specific lactate dehydrogenase. J Ethnopharmacol 144:201–207
Khan BA, Abraham A, Leelamma S (1995) Haematological and histological studies after curry leaf (Murraya koenigii) & mustard (Brassica juncea) feeding in rats. Indian J Med Res 102:184–186
Khaomek P, Ichino C, Ishiyama A, Sekiguchi H, Namatame M, Ruangrungsi N, Saifah E, Kiyohara H, Otoguro K, Omura S, Yamada H (2008) In vitro antimalarial activity of prenylated flavonoids from Erythrina fusca. J Nat Med 62(2):217–220
Kubo I, Chaudhuri SK, Kubo Y, Sanchez Y, Ogura T, Saito T, Ishikawa H, Haraguchi H (1996) Cytotoxic and antioxidative sesquiterpenoids from Heterotheca inuloides. Planta Med 62:427–430
Kumari P, Sahal D, Jain SK, Chauhan VS (2012) Bioactivity guided fractionation of leaves extract of Nyctanthes arbor tristis (Harshringar) against P. falciparum. PLoS One 7(12):e51714
Lee SJ, Park WH, Moon HI (2009) Bioassay-guided isolation of antiplasmodial anacardic acids derivatives from the whole plants of Viola websteri Hemsl. Parasitol Res 104(2):463–466
Li X, Rieckmann K (1992) A bioassay for derivatives of qinghaosu (artemisinin). Trop Med Parasitol 43:195–196
Li JWH, Vederas JC (2009) Drug discovery and natural products: end of an era or an endless frontier? Science 325:161–165
Li Q, Xie LH, Johnson TO, Si Y, Haeberle AS, Weina PJ (2007) Toxicity evaluation of artesunate and artelinate in Plasmodium berghei-infected and uninfected rats. Trans R Soc Trop Med Hyg 101(2):104–112
Liu CH, Huang HY (2012) Antimicrobial activity of curcumin-loaded myristic acid microemulsions against Staphylococcus epidermidis. Chem Pharm Bull (Tokyo) 60(9):1118–1124
Lourens AC, Reddy D, Baser KH, Viljoen AM, Van Vuuren SF (2004) In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J Ethnopharmacol 95:253–258
Macleod JK, Rasmussen HB (1999) A hydroxy-β-caryophyllene from Pterocaulon serrulatum. Phytochem 50:105–108
Malwal M, Sarin R (2010) Chemical characterization and antimicrobial screening of volatile components of Murraya koenigii (L.) Spreng—an Indian aromatic tree. J Pharm Res 3(8):1782–1784
Mathew N, Ayyanar E, Shanmugavelu S, Muthuswamy K (2013) Mosquito attractant blends to trap host seeking Aedes aegypti. Parasitol Res 112(3):1305–1312
Moore BR, Jago JD, Batty KT (2008) Plasmodium berghei: parasite clearance after treatment with dihydroartemisinin in an asplenic murine malaria model. Exp Parasitol 118:458–467
Morais TR, Romoff P, Fávero OA, Reimão JQ, Lourenço WC, Tempone AG, Hristov AD, Di Santi SM, Lago JH, Sartorelli P, Ferreira MJ (2012) Anti-malarial, anti-trypanosomal, and anti-leishmanial activities of jacaranone isolated from Pentacalia desiderabilis (Vell.) Cuatrec. (Asteraceae). Parasitol Res 110(1):95–101
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Mueller D, Davis RA, Duffy S, Avery VM, Camp D, Quinn RJ (2009) Antimalarial activity of azafluorenone alkaloids from the Australian tree Mitrephora diversifolia. J Nat Prod 72(8):1538–1540
Muller O (2011) Challenges for control and elimination in the 21st century. In: Razum O (ed) Malaria in Africa, vol 60. Peter Lang, Frankfurt, p 193
Ndjonka D, Bergmann B, Agyare C, Zimbres FM, Luersen K, Hensel A, Wrenger C, Liebau E (2012) In vitro activity of extracts and isolated polyphenols from West African medicinal plants against Plasmodium falciparum. Parasitol Res 111(2):827–834
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335
Noedl H, Se Y, Socheat D, Fukuda M (2008) Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620
Onayade OA, Adebajo AC (2000) Composition of the leaf volatile oil of Murraya koenigii growing in Nigeria. J Herbs Spices Med Plants 7(4):59–66
Ondo JP, Lekana-Douki JB, Bongui JB, Zang Edou ES, Zatra R, Toure-Ndouo FS, Elomri A, Lebibi J, Seguin E (2012) In vitro antiplasmodial activity and cytotoxicity of extracts and fractions of Vitex madiensis, medicinal plant of Gabon. Trop Med Int Health 17(3):316–321
Orwa JA, Ngeny L, Mwikwabe NM, Ondicho J, Jondiko IJ (2013) Antimalarial and safety evaluation of extracts from Toddalia asiatica (L) Lam. (Rutaceae). J Ethnopharmacol 145(2):587–590
Peter LT, Anatoli VK (1998) The current global malaria situation. Malaria: parasite biology, pathogenesis and protection. ASM Press, Washington, DC, pp 11–22
Peters W, Portus JH, Robinson BL (1975) The chemotherapy of rodent malaria. XXII. The value of drug-resistant strains of Plasmodium berghei in screening for blood schizontocidal activity. Ann Trop Med Parasitol 69:155–171
Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, Ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NPJ, White NJ, Anderson TJ C, Nosten F (2012) Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966
Raina VK, Lal RK, Tripathi S, Khan M, Syamasundar KV, Srivastava SK (2002) Essential oil composition of genetically diverse stocks of Murraya koenigii from India. Flav Fragr J 17:144–146
Ramalhete C, Lopes D, Mulhovo S, Molnar J, Rosario VE, Ferreira MU (2010) New antimalarials with a triterpenic scaffold from Momordica balsamina. Bioorganic Med Chem 18:5254–5260
Rana VS, Juyal JP, Rashmi Blazquez MA (2004) Chemical constituents of the volatile oil of Murraya koenigii leaves. Int J Aromather 14:23–25
Rao LJM, Ramalakshmi K, Borse BB, Raghavan B (2007) Antioxidant and radical scavenging carbazole alkaloids from the oleoresin of curry leaf (Murraya koenigii Spreng). Food Chem 100:742–747
Rukunga GM, Muregi FW, Tolo FM, Omar SA, Mwitari P, Muthaura CN, Omlin F, Lwande W, Hassanali A, Githure J, Iraqi FW, Mungai GM, Kraus W, Kofi-Tsekpo WM (2007) The antiplasmodial activity of spermine alkaloids isolated from Albizia gummifera. Fitoterapia 78(7–8):455–459
Sathaye S, Bagul Y, Gupta S, Kaur H, Redkar R (2011) Hepatoprotective effects of aqueous leaf extract and crude isolates of Murraya koenigii against in vitro ethanol induced hepatotoxicity model. Exp Toxicol Pathol 63:587–591
Severino VG, Cazal Cde M, Forim MR, da Silva MF, Rodrigues-Filho E, Fernandes JB, Vieira PC (2009) Isolation of secondary metabolites from Hortia oreadica (Rutaceae) leaves through high-speed counter-current chromatography. J Chromatogr A 1216(19):4275–4281
Shakya A (2012) Antimalarial activity of Acacia nilotica plant on Plasmodium berghei in mice. IJGHC 1(2):145–150
Shuaibu MN, Wuyep PA, Yanagi T, Hirayama K, Tanaka T, Kouno I (2008) The use of microfluorometric method for activity-guided isolation of antiplasmodial compound from plant extracts. Parasitol Res 102(6):1119–1127
Silva LFRE, Pinto ACS, Pohlit AM, Quignard EL, Vieira PP, Tadei WP, Chaves FC, Samonek JF, Lima CA, Costa MR, Alecrim M, Andrade-Neto VF (2011) In vivo and in vitro antimalarial activity of 4-nerolidylcatechol. Phytother Res 25(8):1181–1188
Simonsen HT, Nordskjold JB, Smitt UW, Nyman U, Palpu P, Joshi P, Varughese G (2001) In vitro screening of Indian medicinal plants for antiplasmodial activity. J Ethnopharmacol 74(2):195–204
Singh G, Marimuthu P, de Heluani CS, Catalan CA (2006) Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components. J Agric Food Chem 54:174–181
Sivakumar R, Jebanesan A, Govindarajan M, Rajasekar P (2011) Larvicidal and repellent activity of tetradecanoic acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) (Diptera: Culicidae). Asian Pac J Trop Med 4(9):706–710
Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M (2004) Simple and inexpensive fluorescence-based technique for high throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806
Son IH, Chung IM, Lee SJ, Moon HI (2007) Antiplasmodial activity of novel stilbene derivatives isolated from Parthenocissus tricuspidata from South Korea. Parasitol Res 101(1):237–241
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193:673–675
Traore M, Diallo A, Nikiema JB, Tinto H, Dakuyo ZP, Ouedraogo JB, Guissou IP, Guiguemde TR (2008) In vitro and in vivo antiplasmodial activity of ‘saye’, an herbal remedy used in Burkina Faso traditional medicine. Phytother Res 22(4):550–551
Tundis R, Loizzo MR, Bonesi M, Menichini F, Dodaro D, Passalacqua NG, Statti G, Menichini F (2009) In vitro cytotoxic effects of Senecio stabianus Lacaita (Asteraceae) on human cancer cell lines. Nat Prod Res 23(18):1707–1718
Walde SG, Jyothirmayi T, Rao PGP, Srinivas P (2006) Flavour volatiles of flowers and stalks of Murraya koenigii L. Flav Fragr J 21:581–584
WHO (World Health Organization) (2012) World malaria report. World Global Malaria Program. http://www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_full_report.pdf
Willcox M, Bodeker R, Rasoanaivo P (2004) Traditional herbal medicines for modern times. Traditional medicinal plants and malaria. CRC, Boca Raton, p 431
Zhang WP, Ruan WB, Deng YY, Gao YB (2012) Potential antagonistic effects of nine natural fatty acids against Meloidogyne incognita. J Agric Food Chem 60(46):11631–11637
Zofou D, Kengne AB, Tene M, Ngemenya MN, Tane P, Titanji VP (2011) In vitro antiplasmodial activity and cytotoxicity of crude extracts and compounds from the stem bark of Kigelia africana (Lam.) Benth (Bignoniaceae). Parasitol Res 108(6):1383–1390
Zofou D, Tene M, Tane P, Titanji VP (2012) Antimalarial drug interactions of compounds isolated from Kigelia africana (Bignoniaceae) and their synergism with artemether, against the multidrug-resistant W2mef Plasmodium falciparum strain. Parasitol Res 110(2):539–544
Acknowledgments
The authors are grateful to C. Abdul Hakeem of the College Management and Dr. S. Y. Anver Sheriff, Principal and Dr. Hameed Abdul Razack, HOD of Zoology Department for providing the facilities to carry out this work. We thank the Management of VIT University, Vellore for providing necessary spectral analysis facilities to carry out this study. We are thankful to Dr. C.R. Pillai, Emeritus Medical Scientist, National Institute of Malaria Research, Delhi, India for providing the Plasmodium berghei (NK65) strain. Chinnaperumal Kamaraj gratefully thanks CSIR, New Delhi for Senior Research Fellowship (CSIR Sc. No. 8/524 (0005)/2011EMR-1).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kamaraj, C., Rahuman, A.A., Roopan, S.M. et al. Bioassay-guided isolation and characterization of active antiplasmodial compounds from Murraya koenigii extracts against Plasmodium falciparum and Plasmodium berghei . Parasitol Res 113, 1657–1672 (2014). https://doi.org/10.1007/s00436-014-3810-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3810-3