Abstract
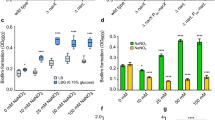
The Bacillus subtilis strain A1/3 shows exceptionally diverse antibiotic capacities compared to other B. subtilis strains. To analyze this phenomenon, mutants for the putative pantotheinyltransferase gene ( pptS), and for several genes involved in non-ribosomal peptide synthesis and polyketide synthesis were constructed and characterized, using bioassays with blood cells, bacterial and fungal cells, and mass spectrometry. Among at least nine distinct bioactive compounds, five antibiotics and one siderophore activity were identified. The anti-fungal and hemolytic activities of strain A1/3 could be eliminated by mutation of the fen and srf genes essential for the synthesis of fengycins and surfactins. Both pptS - and dhb -type mutants were defective in iron uptake, indicating an inability to produce a 2,3-dihydroxybenzoate-type iron siderophore. Transposon mutants in the malonyl CoA transacylase gene resulted in the loss of hemolytic and anti-fungal activities due to the inhibition of bacillomycin L synthesis, and this led to the discovery of bmyLD-LA-LB* genes. In mutants bearing disruption mutations in polyketide ( pksM - and/or pksR -like) genes, the biosynthesis of bacillaene and difficidins, respectively, was inactivated and was accompanied by the loss of discrete antibacterial activities. The formation of biofilms (pellicles) was shown to require the production of surfactins, but no other lipopeptides, indicating that surfactins serve specific developmental functions.









Similar content being viewed by others
References
Battchikova N, Koivulehto M, Denesyuk A, Ptitsyn L, Boretsky Y, Hellman J, Korpela T (1996) Aspartate amino transferase from an alkalophilic Bacillus contains an additional 20-aa extension at its functionally important N-terminus. J Biochem 120:425–432
Behnke D, Gilmore M (1981) Location of antibiotic resistance determinants, copy control, and replication functions on the double-selective streptococcal cloning vector pGB301. Mol Gen Genet 184:115–120
Bernhard F, Demel G, Soltani K, Dohren HV, Blinov V (1996) Identification of genes encoding for peptide synthetases in the gram-negative bacterium Lysobacter sp. ATCC 53042 and the fungus Cylindrotrichum oligospermum. DNA Seq 6:319–330
Besson F, Peypoux F, Michel G, Delcambe L (1978) Identification of antibiotics of iturin group in various strains of Bacillus subtilis. J Antibiot 31:284–288
Borchert S, Patil SS, Marahiel MA (1992) Identification of putative multifunctional peptide synthetase genes using highly conserved oligonucleotide sequences derived from known synthetases. FEMS Microbiol Lett 71:175–180
Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R (2001) Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci USA 98:11621–11626
Chu HH, Hoang V, Kreutzmann P, Hofemeister B, Melzer M, Hofemeister J (2002) Identification and properties of type I-signal peptidases of Bacillus amyloliquefaciens. Eur J Biochem 269:458–469
Conrad B, Bashkirov V, Hofemeister J (1992) Imprecise excision of plasmid pE194 from the chromosomes of Bacillus subtilis pE194 insertion strains. J Bacteriol 174:6997–7002
Duitman EH, Hamoen LW, Rembold M, Venema G, Seitz H, Saenger W, Bernhard F, Reinhardt R, Schmidt M, Ullrich C, Stein T, Leenders F, Vater J (1999) The mycosubtilin synthetase of Bacillus subtilis ATCC6633: a multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc Natl Acad Sci USA 96:13294–13299
Elsner A, Engert H, Saenger W, Hamoen L, Venema G, Bernhard F (1997) Substrate specificity of hybrid modules from peptide synthetases. J Biol Chem 272:4814–4819
Eshita SM, Roberto NH, Beale JM, Mamiya BM, Workman RF (1995) Bacillomycin Lc, a new antibiotic of the iturin group: isolations, structures, and antifungal activities of the congeners. J Antibiot 48:1240–1247
Griesbach E, Lattauschke E (1991) Übertragung von Clavibacter michiganensis subsp. michiganensis in Tomaten-Hydroponikkulturen und Möglichkeiten zur Bekämpfung des Erregers. Nachrichtenbl Deut Pflanzenschutzd 43:69–73
Horionuchi S, Weisblum B (1982) Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J Bacteriol 150:804–814
Huang CC, Ano T, Shoda M (1993) Nucleotide sequence and characteristics of the gene, lpa-14 , responsible for biosynthesis of the lipopeptide antibiotics iturin A and surfactin from Bacillus subtilis RB14. J Ferment Bioeng 76:445–450
Huber J, Griesbach E, Pippig R, Kegler H, Richter S (1991) Mikroorganismus und dessen Verwendung als mikrobielles Pflanzenstärkungsmittel. DD 295 526
Kakinuma A, Hori M, Isono M, Tamura G, Arima K (1969) Determination of amino acid sequence in surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis. Agr Biol Chem 33:971–972
Koumoutsi A, Chen XH, Henne A, Liesegang H, Hitzeroth G, Franke P, Vater J, Borriss R (2004) Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J Bacteriol 186:1084–1096
Kowall M, Vater J, Kluge B, Stein T, Franke P, Ziessow (1998) Separation and characterization of surfactin isoforms produced by Bacillus subtilis OKB 105. J Colloid Interface Sci 203:1–8
Kunst F et al. (1997) The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249–256
Lambalot RH, Gehring AM, Flugel RS, Zuber P, LaCelle M, Marahiel MA, Reid R, Khosla C, Walsh CT (1996) A new enzyme superfamily—the phosphopanthetheinyl transferases. Chem Biol 3:923–936
Landy M, Warren GH, Rosenman SB, Colio LG (1948) Bacillomycin, an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc Soc Exp Biol & Med 67:539–541
Lee SY, Rhee SK, Kim CH, Suh JW (1998) Rapid and efficient isolation of genes for biosynthesis of peptide antibiotics from Gram-positive bacterial strains. J Microbiol Biotechnol 8:310–317
Leenders F, Vater J, Stein T, Franke P (1998) Characterization of the binding site of the tripeptide intermediate D-phenylalanyl L-prolyl-L-valine in gramicidin S biosynthesis. J Biol Chem 273:18011–18014
Leenders F, Stein TH, Kablitz B, Franke P, Vater J (1999) Rapid typing of Bacillus subtilis strains by their secondary metabolites using matrix-assisted laser desorption/ionization mass spectrometry of intact cells. Rapid Commun Mass Spectrom 13:943–949
Lin TP, Chen CL, Chang LK, Tschen JS, Liu ST (1999) Functional and transcriptional analyses of a fengycin synthetase gene, fenC , from Bacillus subtilis. J Bacteriol 181:5060–5067
Marahiel MA, Stachelhaus T, Mootz HD (1997) Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem Rev 97:2651–2674
May JJ, Wendrich TM, Marahiel MA (2001) The dhb operon of Bacillus subtilis encodes the biosynthetic template for the catecholic siderophore 2,3-dihydroxybenzoate-glycine-threonine trimeric ester bacillibactin. J Biol Chem 276:7209–7217
Menkhaus M, Ullrich C, Kluge B, Vater J, Vollenbroich D, Kamp RM (1993) Structural and functional organization of the surfactin synthetase multienzyme system. J Biol Chem 268:7678–7684
Mootz HD, Finking R, Marahiel MA (2001) 4′-phosphopantetheine transfer in primary and secondary metabolism of Bacillus subtilis. J Biol Chem 276:37289–37298
Nakano MM, Corbell N, Besson J, Zuber P (1992) Isolation and characterisation of sfp: a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis. Mol Gen Genet 232:313–321
Neilan BA, Dittmann E, Rouhiainen L, Bass RA, Schaub V, Sivonen K, Börner T (1999) Nonribosomal peptide synthesis and toxigenicity of cyanobacteria. J Bacteriol 181:4089–4097
Pabel CT, Vater J, Wilde C, Franke P, Hofemeister J, Adler B, Bringmann G, Hacker J, Hentschel U (2003) Antimicrobial activities and matrix-assisted laser desorption/ionization mass spectrometry of Bacillus isolates from the marine sponge Aplysina aerophoba. Mar Biotechnol 5:424–434
Patel PS, Huang S, Fisher S, Pirnik D, Aklonis C, Dean L, Meyers E, Fernandes P, Mayerl F (1995) Bacillaene, a novel inhibitor of procaryotic protein synthesis produced by Bacillus subtilis: production, taxonomy, isolation, physico-chemical characterization and biological activity. J Antibiot 48:997–1003
Petit MA, Bruand C, Janniere L, Ehrlich SD (1990) Tn10-derived transposons active in Bacillus subtilis. J Bacteriol 172:6736–6740
Peypoux F, Pommier MT, Das BC, Besson F, Delcambe L, Michel G (1984) Structures of bacillomycin D and bacillomycin L peptidolipid antibiotics from Bacillus subtilis. J Antibiot 37:1600–1604
Schwartz D, Alijah R, Nussbaumer B, Pelzer S, Wohlleben W (1996) The peptide synthetase gene phsA from Streptomyces viridochromogenes is not juxtaposed with other genes involved in nonribosomal biosynthesis of peptides. Appl Environ Microbiol 62:570–577
Seow KT, Meurer G, Gerlitz M, Wendt-Pienkowski E, Hutchinson CR, Davies J (1997) A study of interactive type II polyketide synthases, using bacterial genes cloned from soil DNA: a means to access and use genes from uncultured microorganisms. J Bacteriol 179:7360–7368
Shoda M (2000) Bacterial control of plant disease. J Biosci Bioeng 89:515–521
Sinclair JB (1989) Bacillus subtilis as a biocontrol agent for plant diseases. In: Agrihotri VP, Singh N, Chaube HS, Sinh US, Dwivedi TS (eds) Perspectives in plant pathology. Today and Tomorrow’s Printers and Publishers, New Delhi, pp 367–374
Sokolov SL, Zakharov MV, Esikova TZ, Evdokimova EG, Alakhov YB (2002) Identification and cloning of peptide synthetase genes of thermostable bacilli using the polymerase chain reaction. Russ J Genet 38:1614–1620
Sosio M, Bossi E, Bianchi A, Donadio S (2000) Multiple peptide synthetase gene clusters in Actinomycetes. Mol Gen Genet 264:213–221
Stachelhaus T, Mootz HD, Marahiel MA (2002) Nonribosomal assembly of peptide antibiotics on modular protein templates. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington D.C., pp 415–435
Stein T, Borchert S, Conrad B, Feesche J, Hofemeister B, Hofemeister J, Entian KD (2002) Two different lantibiotic-like peptides originate from the ericin gene cluster of Bacillus subtilis A1/3. J Bacteriol 184:1703–1711
Steinborn G (1998) Zweifach positiv selektive Klonierungsvektoren. DE 196 54 841
Steinborn G, Hofemeister J (1984) Verfahren zur Herstellung von alpha-Amylase. DD B5 233852
Steinborn G, Hofemeister J (1998/2000) Genes for the biosynthesis of anticapsin and bacilysin, their isolation and use. PCT/DE99/02179, WO 00/03009
Steinmetz M, Richter R (1994) Easy cloning of mini-Tn10 insertions from the Bacillus subtilis chromosome. J Bacteriol 176:1761–1763
Steller S, Vollenbroich D, Leenders F, Stein T, Conrad B, Hofemeister J, Jacques P, Thonard P, Vater J (1999) Structural and functional organization of the fengycin synthetase multienzyme system from Bacillus subtilis b213 and A1/3. Chem Biol 6:31–41
Tsuge K, Akiyama T, Shoda M (2001) Cloning, sequencing, and characterization of the iturin A operon. J Bacteriol 183:6265–6273
Turgay K, Marahiel MA (1994) A general approach for identifying and cloning peptide synthetase genes. Pept Res 7:238–241
Uozumi T, Hoshino T, Miwa K, Horinouchi S, Beppu T, Arima K (1977) Restriction and modification in Bacillus species. Genetic transformation of bacteria with DNA from different species, part I. Mol Gen Genet 152:65–69
Vanittanakom N, Loeffler W, Koch U, Jung G (1986) Fengycin—a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J Antibiot 39:888–901
Vater J, Stein T, Vollenbroich D, Kruft V, Wittmann-Liebold B, Franke P, Liu L, Zuber P (1997) The modular organization of multifunctional peptide synthetases. J Protein Chem 16:557–564
Vater J, Kablitz B, Wilde C, Franke P, Mehta N, Cameotra SS (2002) Matrix-assisted laser desorption ionization-time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl Environ Microbiol 68:6210–6219
Vater J, Gao X, Hitzeroth G, Wilde C, Franke P (2003) Whole cell-matrix-assisted laser desorption ionization-time of flight-mass spectrometry, an emerging technique for efficient screening of biocombinatorial libraries of natural compounds—present state of research. CCHTS 6:557–567
Wilson KE, Flor JE, Schwartz RE, Joshua H, Smith JL, Pelak BA, Liesch JM, Hensens OD (1987) Difficidin and oxydifficidin: novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis. II. Isolation and physico-chemical characterization. J Antibiot 40:1682–1691
Yao S, Gao X, Fuchsbauer N, Hillen W, Vater J, Wang J (2003) Cloning, sequencing, and characterization of the genetic region relevant to biosynthesis of the lipopeptides iturin A and surfactin in Bacillus subtilis. Curr Microbiol 47:272–277
Zimmerman SB, Schwartz CD, Monaghan RL, Pelak BA, Weissberger B, Gilfillan EC, Mochales S, Hernandez S, Currie SA, Tejera E (1987) Difficidin and oxydifficidin: novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis. I. Production, taxonomy and antibacterial activity. J Antibiot 40:1677–1681
Zuber P, Nakano MM, Marahiel MA (1993) Peptide antibiotics. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and other Gram-positive bacteria: biochemistry, physiology, and molecular genetics. ASM Press, Washington D.C., pp 897–916
Zweerink MM, Edison A (1987) Difficidin and oxydifficidin: novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis. III. Mode of action of difficidin. J Antibiot 40:1692–1697
Acknowledgements
Strain B. subtilis A1/3 was isolated and provided by Erika Griesbach of the Bundesanstalt für Züchtungsforschung an Kulturpflanzen, Aschersleben. We thank the following collaborators at the IPK, Gatersleben: Susanne König for DNA sequencing, Hans-Wolfgang Jank for computer affairs, and Silke Gorgulla, Ingelore Dommes and Birgit Fischer for expert technical assistance. We are much obliged to Birgit Krebs (FZB Biotechnik GmbH, Berlin) for helpful comments on antibiotic testing. Thanks also to Bettina Kempf and Erhard Bremer (Philipps-Universität Marburg) for providing the plasmid pIC333. The studies in Gatersleben were supported by Grant No. BEO22/0311137 from the Bundesministerium für Bildung und Forschung (BMBF, Germany)
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Kondorosi
Rights and permissions
About this article
Cite this article
Hofemeister, J., Conrad, B., Adler, B. et al. Genetic analysis of the biosynthesis of non-ribosomal peptide- and polyketide-like antibiotics, iron uptake and biofilm formation by Bacillus subtilis A1/3. Mol Genet Genomics 272, 363–378 (2004). https://doi.org/10.1007/s00438-004-1056-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-004-1056-y