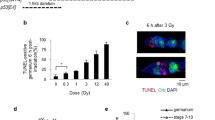
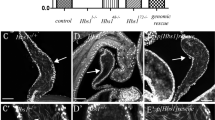
Abstract
p53 family members have been implicated in regulation of genomic integrity and apoptosis in a variety of tissues. The Drosophila family member, Dmp53, primarily functions to regulate apoptosis in developing and regenerating tissues but loss of function mutants are viable and fertile. Dmp53 exhibits a striking expression pattern in the male germline with high levels found in nuclear bodies in pre-meiotic germ cells. The localisation of Dmp53 to nuclear bodies is dependent upon Dmp53 complexes being able to bind DNA, and although dmp53 mutants do not affect germline stem cell (GSC) maintenance or differentiation, GSCs are sensitive to overexpression of Dmp53 but maturing spermatogonia are not. Dmp53 thus has differential effects depending upon the stage of male germline maturation.





Similar content being viewed by others
References
Bauer JH, Poon PC, Glatt-Deeley H, Abrams JM, Helfand SL (2005) Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends life span. Curr Biol 15:2063–2068
Bauer JH, Chang C, Morris SN, Hozier S, Andersen S, Waitzman JS, Helfand SL (2007) Expression of dominant-negative Dmp53 in the adult fly brain inhibits insulin signaling. Proc Natl Acad Sci USA 104:13355–13360
Bernassola F, Oberst A, Melino G, Pandolfi PP (2005) The promyelocytic leukaemia protein tumour suppressor functions as a transcriptional regulator of p63. Oncogene 24:6982–6986
Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM (2000) Drosophila p53 binds a damage response element at the reaper locus. Cell 101:103–113
Brodsky MH, Weinert BT, Tsang G et al (2004) Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol 24:1219–1231
Bunt SM, Hime GR (2004) Ectopic activation of Dpp signalling in the male Drosophila germline inhibits germ cell differentiation. Genesis 39:84–93
Carpenter ATC (1975) Electron microscopy of meiosis in Drosophila melanogaster females. I. Structure, arrangement, and temporal change of the synaptonemal complex in wild-type. Chromosoma 51:157–182
Chen D, McKearin DM (2003) A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 130:1159–1170
Deyoung MP, Ellisen LW (2007) p63 and p73 in human cancer: defining the network. Oncogene 26:5169–5183
Fuller MT (1993) Spermatogenesis. In: Harbour CS (ed) The development of Drosophila melanogaster. Cold Spring Harbour Laboratory Press, NY, pp 71–147
Gan Q, Chepelev I, Wei G, Tarayrah L, Cui K, Zhao K, Chen X (2010) Dynamic regulation of alternative splicing and chromatin structure in Drosophila gonads revealed by RNA-seq. Cell Res 20:763–783
Gonczy P, DiNardo S (1996) The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development 122:2437–2447
Gonczy P, Matunis E, DiNardo S (1997) Bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development 124:4361–4371
Gostissa M, Hofmann TG, Will H, Del Sal G (2003) Regulation of p53 functions: let’s meet at the nuclear bodies. Curr Opin Cell Biol 15:351–357
Harris RE, Pargett M, Sutcliffe C, Umulis D, Ashe HL (2011) Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev Cell 20:72–83
Hasegawa M, Zhang Y, Niibe H, Terry NH, Meistrich ML (1998) Resistance of differentiating spermatogonia to radiation-induced apoptosis and loss in p53-deficient mice. Radiat Res 149:263–270
Hay RT (2005) SUMO: a history of modification. Mol Cell 18:1–12
Insco ML, Leon A, Tam CH, McKearin DM, Fuller MT (2009) Accumulation of a differentiation regulator specifies transit amplifying division number in an adult stem cell lineage. Proc Natl Acad Sci USA 106:22311–22316
Jassim OW, Fink JL, Cagan RL (2003) Dmp53 protects the Drosophila retina during a developmentally regulated DNA damage response. EMBO J 22:5622–5632
Jin S, Martinek S, Joo WS, Wortman JR, Mirkovic N, Sali A, Yandell MD, Pavletich NP, Young MW, Levine AJ (2000) Identification and characterization of a p53 homologue in Drosophila melanogaster. Proc Natl Acad Sci USA 97:7301–7306
Lallemand-Breitenbach V, de The H (2010) PML nuclear bodies. Cold Spring Harb Perspect Biol 2:a000661
LaRocque JR, Dougherty DL, Hussain SK, Sekelsky J (2007) Reducing DNA polymerase alpha in the absence of Drosophila ATR leads to P53-dependent apoptosis and developmental defects. Genetics 176:1441–1451
Lavoie CA, Ohlstein B, McKearin DM (1999) Localization and function of Bam protein require the benign gonial cell neoplasm gene product. Dev Biol 212:405–413
Leatherman JL, DiNardo S (2010) Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol 12:806–811
Lee JH, Lee E, Park J, Kim E, Kim J, Chung J (2003) In vivo p53 function is indispensable for DNA damage-induced apoptotic signaling in Drosophila. FEBS Lett 550:5–10
Li Y, Jenkins CW, Nichols MA, Xiong Y (1994) Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene 9:2261–2268
Lindsley DL, Tokuyasu KT (1980) Spermatogenesis. In: Ashburner M, Wright TRF (eds) The genetics and biology of Drosophila. Academic, London, pp 225–294
Liu N, Han H, Lasko P (2009) Vasa promotes Drosophila germline stem cell differentiation by activating mei-P26 translation by directly interacting with a (U)-rich motif in its 3′ UTR. Genes Dev 23:2742–2752
Lu WJ, Chapo J, Roig I, Abrams JM (2010) Meiotic recombination provokes functional activation of the p53 regulatory network. Science 328:1278–1281
Lunardi A, Chiacchiera F, D’Este E et al (2009) The evolutionary conserved gene C16orf35 encodes a nucleo-cytoplasmic protein that interacts with p73. Biochem Biophys Res Commun 388:428–433
Marcel V, Dichtel-Danjoy ML, Sagne C et al (2011) Biological functions of p53 isoforms through evolution: lessons from animal and cellular models. Cell Death Differ 18:1815–1824
Mauri F, McNamee LM, Lunardi A, Chiacchiera F, Del Sal G, Brodsky MH, Collavin L (2008) Modification of Drosophila p53 by SUMO modulates its transactivation and pro-apoptotic functions. J Biol Chem 283:20848–20856
McKearin DM, Spradling AC (1990) Bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev 4:2242–2251
Mehrotra S, Maqbool SB, Kolpakas A, Murnen K, Calvi BR (2008) Endocycling cells do not apoptose in response to DNA rereplication genotoxic stress. Genes Dev 22:3158–3171
Morgan TH (1914) No crossing over in the male of Drosophila of genes in the second and third pairs of chromosomes. Biol Bull (Woods Hole) 26:195–204
Nakano K, Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7:683–694
Ollmann M, Young LM, Di Como CJ et al (2000) Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell 101:91–101
Oswald C, Stiewe T (2008) In good times and bad: p73 in cancer. Cell Cycle 7:1726–1731
Petre-Lazar B, Livera G, Moreno SG et al (2007) The role of p63 in germ cell apoptosis in the developing testis. J Cell Physiol 210:87–98
Rosenbluth JM, Pietenpol JA (2008) The jury is in: p73 is a tumor suppressor after all. Genes Dev 22:2591–2595
Rotter V, Schwartz D, Almon E et al (1993) Mice with reduced levels of p53 protein exhibit the testicular giant-cell degenerative syndrome. Proc Natl Acad Sci USA 90:9075–9079
Rutkowski R, Hofmann K, Gartner A (2010) Phylogeny and function of the invertebrate p53 superfamily. Cold Spring Harb Perspect Biol 2:a001131
Sogame N, Kim M, Abrams JM (2003) Drosophila p53 preserves genomic stability by regulating cell death. Proc Natl Acad Sci USA 100:4696–4701
Spradling A, Drummond-Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414:98–104
Stiewe T (2007) The p53 family in differentiation and tumorigenesis. Nat Rev Cancer 7:165–168
Suh EK, Yang A, Kettenbach A et al (2006) p63 protects the female germ line during meiotic arrest. Nature 444:624–628
Sutcliffe JE, Brehm A (2004) Of flies and men; p53, a tumour suppressor. FEBS Lett 567:86–91
Terry NA, Tulina N, Matunis E, DiNardo S (2006) Novel regulators revealed by profiling Drosophila testis stem cells within their niche. Dev Biol 294:246–257
Titen SW, Golic KG (2008) Telomere loss provokes multiple pathways to apoptosis and produces genomic instability in Drosophila melanogaster. Genetics 180:1821–1832
Tomasini R, Mak TW, Melino G (2008a) The impact of p53 and p73 on aneuploidy and cancer. Trends Cell Biol 18:244–252
Tomasini R, Tsuchihara K, Wilhelm M et al (2008b) TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev 22:2677–2691
Tomasini R, Tsuchihara K, Tsuda C et al (2009) TAp73 regulates the spindle assembly checkpoint by modulating BubR1 activity. Proc Natl Acad Sci USA 106:797–802
Van Doren M, Williamson AL, Lehmann R (1998) Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol 8:243–246
van Leeuwen F, van Steensel B (2005) Histone modifications: from genome-wide maps to functional insights. Genome Biol 6:113
Wells BS, Yoshida E, Johnston LA (2006) Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol 16:1606–1615
Yamada Y, Davis KD, Coffman CR (2008) Programmed cell death of primordial germ cells in Drosophila is regulated by p53 and the outsiders monocarboxylate transporter. Development 135:207–216
Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L (2003) PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA 100:1931–1936
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monk, A.C., Abud, H.E. & Hime, G.R. Dmp53 is sequestered to nuclear bodies in spermatogonia of Drosophila melanogaster . Cell Tissue Res 350, 385–394 (2012). https://doi.org/10.1007/s00441-012-1479-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-012-1479-4