Abstract
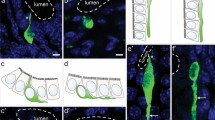
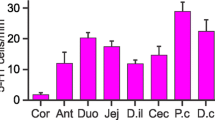
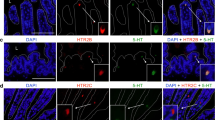
Serotonin (5-HT)-containing gastrointestinal endocrine cells contribute to regulation of numerous bodily functions, but whether these functions are related to differences in cell shape is not known. The current study identified morphologies and localization of subtypes of 5-HT-containing enteroendocrine cells in the mouse large intestine. 5-HT cells were most frequent in the proximal colon compared with cecum and distal colon. The large intestine harbored both open (O) cells, with apical processes that reached the lumen, and closed (C) cells, not contacting the lumen, classified into O1, O2, and O3 and C1, C2, and C3 cells, by the lengths of their basal processes. O1 and C1 cells, with basal processes sometimes longer that 100 µm, were most common in the distal colon. Their long basal processes ran against the inner surfaces of the mucosal epithelial cells and were strongly immunoreactive for 5-HT; these processes are ideally placed to communicate with the epithelium and to react to mechanical forces. O2 and C2 cells that had similar but shorter basal processes were also most common in the distal colon. O3 and C3 cells had no or very short basal processes. The O3 open type 5-HT cells were abundant in the proximal colon, particularly at the luminal surface, where they could release 5-HT into the lumen to act on luminal 5-HT receptors. Numerous O3 type 5-HT cells occurred in the lower (submucosal) region of the crypts in all segments and might release 5-HT to influence cell renewal in the crypt proliferative zones.





Similar content being viewed by others
References
Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia G, Beyder A (2018) A population of gut epithelial enterochromaffin cells is mechanosensitive and required Piezo2 to convert force into serotonin release. PNAS 115:E7632–E7641
Berger M, Gray JA, Roth BL (2009) The expanded biology of serotonin. Annu Rev Med 60:355–366
Billing LJ, Larraufie P, Lewis J, Leiter A, Li J, Lam B, Yeo GS, Goldspink DA, Kay RG, Gribble FM, Reimann F (2019) Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice–identification of selective stimuli for insulin-like peptide-5 and glucagon-like peptide-1 co-expressing cells. Mol metab 29:158–169
Bohórquez DV, Chandra R, Samsa LA, Vigna SR, Liddle RA (2011) Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Hist 42:3–13
Bülbring E, Crema A (1958) Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. Br J Pharmacol 13:444–457
Bülbring E, Lin RCY (1958) The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol (Lond) 140:381–407
Cooke HJ, Carey HV (1985) Pharmacological analysis of 5-hydroxytryptamine actions on guinea-pig ileal mucosa. Eur J Pharmacol 111:329–337
Cox HM (2016) Neuroendocrine peptide mechanisms controlling intestinal epithelial function. Curr Opin Pharmacol 31:50–56
Day J, King B, Haque SM, Kellum JM (2005) A nonneuronal 5-hydroxytryptamine receptor 3 induces chloride secretion in the rat distal colon mucosa. Am J Surg 190:736–738
de Bruine AP, Dinjens WNM, Zijlema JHL, Lenders M-H, Bosman FT (1992) Renewal of enterochromaffin cells in the rat caecum. Anat Rec 233:75–82
Diwakarla S, Fothergill LJ, Fakhry J, Callaghan B, Furness JB (2017) Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol Motil 29:e13101
Fakhry J, Stebbing MJ, Hunne B, Bayguinov Y, Ward SM, Casse KC, Callaghan B, McQuade RM, Furness JB (2019) Relationships of endocrine cells to each other and to other cell types in the human gastric fundus and corpus. Cell Tissue Res 376:37–49
Fazio Coles TE, Fothergill LJ, Hunne B, Nikfarjam M, Testro A, Callaghan B, McQuade RM, Furness JB (2020) Quantitation and chemical coding of enteroendocrine cell populations in the human jejunum. Cell Tissue Res 379:109–120
Ferrara A, Zinner MJ, Jaffe BM (1987) Intraluminal release of serotonin, substance P, and gastrin in the canine small intestine. Dig Dis Sci 32:289–294
Fujimiya M, Okumiya K, Kuwahara A (1997) Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol 108:105–113
Fujita T, Kobayashi S (1971) Experimental induced granule release in the endocrine cells of dog pyloric antrum. Z Zellforsh 116:52–60
Fujita T, Kobayashi S (1973) The cells and hormones of the GEP endocrine system—the current of studies. In: Fujita T (ed) Gasro-entero-pancreatic endocrine system: a cell-biological approach. Igaku-Shoin, Tokyo, pp 1–16
Fujita T, Kanno T, Kobayashi S (1988) Gastroenteropancreatic endocrine system. In: Fujita T, Kanno T, Kobayashi S (eds) The paraneuron. Springer-Verlag, Tokyo, pp 165–184
Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T (2003) Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 284:R1269–R1276
Furness JB, Rivera LR, Cho H-J, Bravo DM, Callaghan B (2013) The gut as a sensory organ. Nat Rev Gastroenterol Hepatol 10:729–740
Greig CJ, Zhang L, Cowles (2019) Potentiated serotonin signaling in serotonin re-uptake transporter knockout mice increases enterocyte mass and small intestinal absorptive function. Physiol Rep 7:e14278–e14286
Gribble FM, Reimann F (2016) Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol 78:277–299
Grider JR, Kuemmerle JF, Jin JG (1996) 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol 270:G778–G782
Hansen MB, Witte A-B (2008) The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol 193:311–323
Hunne B, Stebbing MJ, McQuade RM, Furness JB (2019) Distributions and relationships of chemically defined enteroendocrine cells in the rat gastric mucosa. Cell Tissue Res 378:33–48
Kaji I, Akiba Y, Said H, Narimatsu K, Kaunitz JD (2015) Luminal 5-HT stimulates colonic bicarbonate secretion in rats. Br J Pharmacol 172:4655–4670
Kellum J, McCabe M, Scheiei J, Donomitz M (1983) Neural control of acid-induced serotonin release from rabbit duodenum. Am J Physiol 245:G824–G831
Kobayashi S, Fujita T, Sasagawa T (1971) Electron microscope studies on the endocrine cells of the human gastric fundus. Arch Histol Jpn 31:429–444
Kojima S, Ikeda M (1998) Facilitation by endogenous acetylcholine and nitric oxide of luminal serotonin release from the guinea-pig colon. Eur J Pharmacol 355:51–55
Kuramoto H, Kadowaki M, Sakamoto H, Yuasa K, Todo A, Shirai R (2007) Distinct morphology of serotonin-containing enterochromaffin (EC) cells in the rat distal colon. Arch Histol Cytol 70:235–241
Larsson LI, Goltermann N, De Magistris L, Rehfeld JF, Schwarz TW (1979) Somatostatin cell processes as pathways for paracrine secretion. Science 205:1393–1395
Lund ML, Egerod KL, Engelstoft MS, Dmytriyeva O, Theodorsson E, Patel BA, Schwartz TW (2018) Enterochromaffin 5-HT cells - a major target for GLP-1 and gut microbial metabolites. Mol Metab 11:70–83
Lundberg JM, Tatemoto K, Terenius L, Hellström PM, Mutt V, Hökfelt T, Hamberger B (1982) Localization of peptide YY PYY in gastrointestinal endocrine cells and effects on intestinal blood flow and motility. Proc Natl Acad Sci USA 79:4471–4475
Martin AM, Lumsden AL, Young RL, Jessup CF, Spencer NJ, Keating DJ (2017) The nutrient-sensing repertoires of mouse enterochromaffin cells differ between duodenum and colon. Neurogastroenterol Motil 29:e13046
Martin AM, Young RL, Leong L, Rogers GB, Spencer NJ, Jessup CF, Keating DJ (2017) The diverse metabolic roles of peripheral serotonin. Endocrinology 158:1049–1063
Reynaud Y, Fakhry J, Fothergill L, Callaghan B, Ringuet MT, Hunne B, Bravo DM, Furness JB (2016) The chemical coding of 5-hydroxytryptamine containing enteroendocrine cells in the mouse gastrointestinal tract. Cell Tissue Res 364:489–497
Ripken D, Wielen Nvd, Wortelboer HM, Meijerink J, Witkamp RF, Hendriks HFJ (2016) Nutrient-induced glucagon like peptide-1 release is modulated by serotonin. J Nutr Bichem 32:142–150
Schubert ML, Peura DA (2008) Control of gastric acid secretion in health and disease. Gasroenterology 134:1842–1860
Spohn SN, Mawe GM (2017) Non-conventional features of peripheral serotonin signalling — the gut and beyond. Nat Rev Gastroenterol Hepatol
Tackett JJ, Gandotra N, Bamdad MC, Muise ED, Cowles RA (2017) Enhanced serotonin signaling stimulates ordered intestinal mucosal growth. J Surg Res 208:198–203
Tackett JJ, Gandotra N, Bamdad MC, Muise ED, Cowles RA (2019) Potentiation of serotonin signaling protects against intestinal ischemia and reperfusion injury in mice. Neurogastroenterol Motil 31:e13498–e13507
Tsubouchi S, Leblond CP (1979) Migration and turnover of entero-endocrine and caveated cells in the epithelium of the descending colon, as shown by radioautography after continuous infusion of 3H-thymidine into mice. Am J Anat 156:431–452
Tsukamoto K, Ariga H, Mantyh C, Pappas TN, Yanagi H, Yamamura T, Takahashi T (2007) Luminally released serotonin stimulates colonic motility and accelerates colonic transit in rats. Am J Physiol 293:R64–R69
Tutton PJ, Barkla DH (1986) Serotonin receptors influencing cell proliferation in the jejunal crypt epithelium and in colonic adenocarcinomas. Anticancer Res 6:1123–1126
Vialli M, Erspamer V (1933) Celluli enterocromaffini e cellule basigranulose acidofile nei vertebrati. Z Zellforsch 19:743–773
Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, Kashyap P, Grover M, Oeckler R, Gottlieb PA, Li HJ, Leiter AB, Farrugia G, Beyder A (2017) Mechanosensitive ion channel piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol 595:79–91
Yurchenko PD, Schittny JC (1990) Molecular architecture of basement membranes. FASEB J 4:1577–1590
Zachrisson K, Uribe A (1998) Serotonin and neuroendocrine peptides influence DNA synthesis in rat and human small intestinal cells in vitro. Acta Physiol Scand 163:195–200
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest:
The authors declare that they have no conflict of interest.
Ethical approval
Procedures were approved by the University of Melbourne Animal Ethics Committee (ethics approval numbers 1613817 and 1814569) and the Kyoto Institute of Technology (approval number 100154). All applicable National and Institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuramoto, H., Koo, A., Fothergill, L.J. et al. Morphologies and distributions of 5-HT containing enteroendocrine cells in the mouse large intestine. Cell Tissue Res 384, 275–286 (2021). https://doi.org/10.1007/s00441-020-03322-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03322-6