Abstract
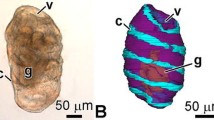
The morphology and regeneration of the digestive system and tegmen after autotomy of the visceral mass in the crinoid Lamprometra palmata (Clark 1921) was studied. The gut has a five-lobed shape and is covered by a tegmen. The tegmen consists of epidermis and underlying connective tissue. The digestive tube can be divided into three parts: esophagus, intestine, and rectum. At 6 h post-autotomy, the calyx surface is covered by a layer of amoebocytes and juxtaligamental cells (JLCs). At 14–18 h, post-autotomy transdifferentiation of JLCs begins and give rise to the epidermis and cells of digestive system. On days 1–2 post-autotomy, JLCs undergo the mesenchymal–epithelial transition. Some JLCs turn into typical epidermal cells, while other JLCs form small closed epithelial structures that represent the gut anlage. On day 4 post-autotomy, the animals have a mouth opening and a small anal cone. On day 7 post-autotomy, the visceral mass and the digestive system become fully formed but are smaller than normal. A 24-h exposure of L. palmata individuals to a 10−7 M colchicine solution did not slow down regeneration, and the timing of gut formation was similar to that in the control animals. We conclude that JLCs are the major cell source for gut and epidermis regeneration in L. palmata. The main mechanisms of morphogenesis are cell migration, mesenchymal–epithelial transition, and transdifferentiation.












Similar content being viewed by others
References
Amemiya S, Oji T (1992) Regeneration in sea lilies. Nature 357(6379):546–547
Atala A (2000) Tissue engineering of artificial organs. J Endourol 14(1):49–57
Bely A (2014) Early events in annelid regeneration: a cellular perspective. Integr Comp Biol 54:688–699
Biressi A, Zou T, Dupont S, Dahlberg C, Di Benedetto C, Bonasoro F, Thorndyke M, Carnevali MD (2010) Wound healing and arm regeneration in Ophioderma longicaudum and Amphiura filiformis (Ophiuroidea, Echinodermata): comparative morphogenesis and histogenesis. Zoomorphology 129(1):1–19
Bobrovskaya NV, Dolmatov IY (2014) Autotomy of the visceral mass in the feather star Himerometra robustipinna (Crinoidea, Comatulida). Biol Bull 226(2):81–91
Borisenko I, Adamska M, Tokina D, Ereskovsky A (2015) Transdifferentiation is a driving force of regeneration in Halisarca dujardini (Demospongiae, Porifera). PeerJ 3:e1211. https://doi.org/10.7717/peerj.1211
Borisov A (1999) Regeneration of skeletal and cardiac muscle in mammals: do nonprimate models resemble human pathology? Wound Repair Regen 7(1):26–35
Byrne M (1994) Ophiuroidea. In: Harrison FW, Chia FS (eds) microscopic anatomy of invertebrates, vol 14. Echinodermata. Wiley-Liss, New York, pp 247–344
Campbell C, Lancman JJ, Palazon RE, Matalonga J, He J, Graves A, Zeng X-XI, Mishra R, Huisken J, Traver D, Dong PDS (2019) In vivo lineage conversion of vertebrate muscle into early endoderm-like cells. bioRxiv 722967
Candia Carnevali MD (2006) Regeneration in echinoderms: repair, regrowth, cloning. Invert Surviv J 3(1):64–76
Cary GA, Wolff A, Zueva O, Pattinato J, Hinman VF (2019) Analysis of sea star larval regeneration reveals conserved processes of whole-body regeneration across the metazoa. BMC Biology 17:16
Carlson BM (2007) Principles of regenerative biology. Academic Press-Elsevier, San Diego, CA
Cavey MJ, Märkel K (1994) Echinoidea. In: Harrison FW, Chia FS (eds) Microscopic anatomy of invertebrates, vol 14. Echinodermata. Wiley-Liss Inc, New York, pp 345–400
Day RC, Beck CW (2011) Transdifferentiation from cornea to lens in Xenopus laevis depends on BMP signalling and involves upregulation of Wnt signalling. BMC Dev Biol 11(1):54
Dietrich HF, Fontaine AR (1975) A decalification method for ultrastructure of echinoderm tissues. Stain Technol 50(5):351–354
Dobson WE, Turner RL (1989) Morphology and histology of the disk autotomy plane in Ophiophragmus filograneus (Echinodermata, Ophiurida). Zoomorphology 108(6):323–332
Dolmatov IY (1992) Regeneration of the aquapharyngeal complex in the holothurian Eupentacta fraudatrix (Holothuroidea, Dendrochirota). In: Taban CH, Boilly B (eds) Keys for Regeneration. Monogr Dev Biol 23. Karger, Basel, pp 40-50
Dolmatov IY (1993) Proliferation of tissues of regenerating aquapharyngeal complex in holothurians. Russ J Dev Biol 24:72–81
Dolmatov IY (1999) Regeneration in echinoderms. Russian J Mar Biol 25(3):191–200
Dolmatov IY (2009) Regeneration of the digestive system in holothurians. Zhurnal Obsh Biol 70(4):316–327
Dolmatov IYu (2020) Variability of regeneration mechanisms in echinoderms. Russ J Mar Biol 46(6):363–376
Dolmatov IY (2021) Molecular aspects of regeneration mechanisms in holothurians. Genes 12:250
Dolmatov IY, Ginanova TT (2001) Muscle regeneration in holothurians. Microsc Res Tech 55:452–463
Dolmatov IY, Ginanova TT (2009) Post-autotomy regeneration of respiratory trees in the holothurian Apostichopus japonicus (Holothuroidea, Aspidochirotida). Cell Tissue Res 336(1):41–58
Dolmatov IY, Ginanova TT, Frolova LT (2016) Metamorphosis and definitive organogenesis in the holothurian Apostichopus japonicus. Zoomorphology 135(2):173–188
Dolmatov IY, Kalacheva NV, Mekhova ES, Frolova LT (2020) Autotomy and regeneration of the visceral mass in feather stars. Zoomorphology. 139:171–187
Eguizabal C, Montserrat N, Veiga A, Belmonte JCI (2013) Dedifferentiation, transdifferentiation, and reprogramming: future irections in regenerative medicine. Semin Reprod Med 31:082–094
Elchaninov AV, Fatkhudinov TC (2020) Regeneration of liver in mammals. Intercellular interactions. Nauka, Moscow
Eliseikina MG, Magarlamov TY, Dolmatov IY (2010) Stem cells of holothuroid coelomocytes. In: Bottger SA, Walker CW, Lesser MP (eds) Harris LG. Durham. CRC Press Boca Raton, Echinoderms, pp 163–166
Ereskovsky AV, Tokina DB, Saidov DM, Baghdiguian S, Le Goff E, Lavrov AI (2020) Transdifferentiation and mesenchymal-to-epithelial transition during regeneration in Demospongiae (Porifera). J Exp Zool Part B 334(1):37–58
Féral JP, Massin C (1982) Digestive systems: Holothuroidea. In: Jangoux M, Lawrence JM (eds) Echinoderm nutrition. Balkema, Rotterdam, pp 191–212
Filimonova GF, Tokin IB (1980) Structural and functional peculiarities of the digestive system of Cucumaria frondosa (Echinodermata: Holothuroidea). Mar Biol 60(1):9–16
Frolova LT, Dolmatov IY (2010) Microscopic anatomy of the digestive system in normal and regenerating specimens of the brittlestar Amphipholis kochii. Biol Bull 218:303–316
Fujiwara S, Kawamura K (1992) Ascidian budding as a transdifferentiation-like system: multipotent epithelium is not undifferentiated. Dev Growth Differ 34:463–472
García-Arrarás J, Estrada-Rodgers L, Santiago R, Torres I, Díaz-Miranda L, Torres-Avillán I (1998) Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea: Echinodermata). J Exp Zool 281(4):288–304
García-Arrarás JE, Bello SA, Malavez S (2019) The mesentery as the epicenter for intestinal regeneration. Semin Cell Dev Biol 92:45–54
García-Arrarás JE, Dolmatov IY (2010) Echinoderms: potential model systems for studies on muscle regeneration. Current Pharm Design 16(8):942–955
García-Arrarás JE, Valentín-Tirado G, Flores JE, Rosa RJ, Rivera-Cruz A, San Miguel-Ruiz JE, Tossas K (2011) Cell dedifferentiation and epithelial to mesenchymal transitions during intestinal regeneration in H. glaberrima. BMC Dev Biol 11(1):1–18
Hasan A (Ed.) (2017) Tissue engineering for artificial organs: regenerative medicine, smart diagnostics and personalized medicine. John Wiley & Sons.
Heinzeller T, Welsch U (1994) Crinoidea. In: Harrison FW, Chia FS (eds) Microscopic anatomy ofinvertebrates, vol 14: Echinodermata. Wiley-Liss Inc, New York, pp 9–148
Hench L, Jones J (ed.) (2005) Biomaterials, artificial organs and tissue engineering. Elsevier
Holland N, Leonard A, Meyer D (1991) Digestive mechanics and gluttonous feeding in the feather star Oligometra serripinna (Echinodermata: Crinoidea). Mar Biol 111:113–119
Jopling C, Boue S, Belmonte JCI (2011) Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol 12(2):79
Kalacheva N, Eliseikina M, Frolova L, Dolmatov I (2017) Regeneration of the digestive system in the crinoid Himerometra robustipinna occurs by transdifferentiation of neurosecretory-like cells PLoS One. 12 https://doi.org/10.1371/journal.pone.0182001
Kalacheva NV, Dolmatov IY (2019) Cellular source of digestive system regeneration in Lamprometra palmata and Anneissia bennetti. Abstracts of 10th European conference on echinoderms, Borissiak Paleontological Institute RAS, Moscow, pp 42
Kamenev YO, Dolmatov IY, Frolova LT, Khang NA (2013) The morphology of the digestive tract and respiratory organs of the holothurian Cladolabes schmeltzii (Holothuroidea, Dendrochirotida). Tissue Cell 45(2):126–139
Kostyuchenko R, Kozin V (2020) Morphallaxis versus epimorphosis? Cellular and molecular aspects of regeneration and asexual reproduction in annelids. Biol Bull 47(3):237–246
Kostyuchenko R, Kozin V, Kupriashova E (2016) Regeneration and asexual reproduction in annelids: cells, genes, and evolution. Biol Bull 43(3):185–194
LaHaye C, Holland N (1984) Electron microscopic studies of the digestive tract and absorption from the gut lumen of a feather star, Oligometra serripinna (Echinodermata). Zoomorphology 104(4):252–259
Maden M (2018) The evolution of regeneration – where does that leave mammals? Int J of Dev Biol 62:369–37
Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Südhof TC, Wernig M (2011) Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell 9:374–382
Mashanov V, Dolmatov I, Heinzeller T (2005) Transdifferentiation in holothurian gut regeneration. Biol Bull 209:184–193
Mashanov V, García-Arrarás J (2011) Gut regeneration in holothurians: a snapshot of recent developments. Biol Bull 221:93–109
Mashanov VS, Charlina NA, Dolmatov IY, Wilkie IC (2007) Juxtaligamental cells in the arm of the brittlestar Amphipholis kochii Lu¨tken, 1872 (Echinodermata: Ophiuroidea). Russian J Mar Biol 33:110–117
Mashanov VS, Dolmatov IY (2001) Regeneration of digestive tract in the pentactulae of the far-eastern holothurian Eupentacta fraudatrix (Holothuroidea, Dendrochirota). Invertebr Reprod Dev 39(2):143–151
Mashanov VS, Frolova LT, Dolmatov IY (2004) Structure of the digestive tube in the holothurian Eupentacta fraudatrix (Holothuroidea: Dendrochirota). Russian J Mar Biol 30(5):314–322
Mashanov VS, Zueva OR, García-Arrarás JE (2017) Inhibition of cell proliferation does not slow down echinoderm neural regeneration. Front Zool 14(1):12
Merrell A, Stanger B (2016) Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat Rev Mol Cell Bio 17:413
Metscher B (2009) MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol 9:11
Morata G, Herrera S (2016) Cell reprogramming during regeneration in Drosophila: transgression of compartment boundaries. Curr Opin Genet Dev 40:11–16
Morgan T (1901) Regeneration and liability to injury. Science 14:235–248
Motokawa T (2011) Mechanical mutability in connective tissue of starfish body wall. Biol Bull 221:280–289
Mozzi D, Dolmatov I, Bonasoro F, Candia Carnevali M (2006) Visceral regeneration in the crinoid Antedon mediterranea: basic mechanisms, tissues and cells involved in gut regrowth. Cent Eur J Biol 1:609–635
Nikanorova D, Kupriashova E, Kostyuchenko R (2020) Regeneration in Annelids: Cell Sources, Tissue Remodeling, and Differential Gene Expression. Russ J Dev Biol 51:148–161
Reddien PW (2018) The cellular and molecular basis for planarian regeneration. Cell 175(2):327–345
Ribeiro AR, Barbaglio A, Benedetto CD, Ribeiro CC, Wilkie IC, Carnevali MD, Barbosa MA (2011) New insights into mutable collagenous tissue: correlations between the microstructure and mechanical state of a sea-urchin ligament. PLoS One 6(9): e24822
Ribeiro AR, Barbaglio A, Oliveira MJ, Ribeiro CC, Wilkie IC, Carnevali MDC, Barbosa MA (2012b) Matrix metalloproteinases in a sea urchin ligament with adaptable mechanical properties. PLoS One 7: e49016.
Ribeiro AR, Barbaglio A, Oliveira MJ, Santos R, Coelho AV, Ribeiro CC, Wilkie MD, Candia Carnevali MD, Barbosa MA (2012) Correlations between the biochemistry and mechanical states of a sea-urchin ligament: a mutable collagenous structure. Biointerphases 7:38
Ribeiro R, Ponz-Segrelles G, Bleidorn C, Aguado T (2019) Comparative transcriptomics in Syllidae (Annelida) indicates that posterior regeneration and regular growth are comparable, while anterior regeneration is a distinct process. BMC Genomics 20:855
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Seifert AW, Muneoka K (2018) The blastema and epimorphic regeneration in mammals. Dev biol 433(2):190–199
Shekhani MT, Jayanthy AS, Maddodi N, Setaluri V (2013) Cancer stem cells and tumor transdifferentiation: implications for novel therapeutic strategies. Am J Stem Cells 2(1):52
Sisakhtnezhad S, Matin MM (2012) Transdifferentiation: a cell and molecular reprogramming process. Cell Tissue Res 348(3):379–396
Stocum D (2012) Regenerative biology and medicine. Academic Press
Sugimoto K, Gordon SP, Meyerowitz EM (2011) Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol 21(4):212–218
Takeo M, Chou WC, Sun Q, Lee W, Rabbani P, Loomis C, Taketo MM, Ito M (2013) Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature 499(7457):228–232
Tanaka EM, Reddien PW (2011) The cellular basis for animal regeneration. Dev Cell 21(1):172–185
Thorndyke MC, Candia Carnevali MD (2001) Regeneration neurohormones and growth factors in echinoderms. Can J Zool 79:1171–1208
Vogt G (2012) Hidden treasures in stem cells of indeterminately growing bilaterian invertebrates. Stem Cell Rev Rep 8:305–317
Vorontsova A, Liozner L (1960) Asexual propagation and regeneration
Wilkie IC (1979) The juxtaligamental cells of Ophiocomina nigra (Abildgaard) (Echinodermata: Ophiuroidea) and their possible role in mechano-effector function of collagenous tissue. Cell Tissue Res 197:515–530
Wilkie IC (2001) Autotomy as a prelude to regeneration in echinoderms. Microsc Res Techniq 55:369–396
Wilkie IC (2016) Functional morphology of the arm spine joint and adjacent structures of the brittlestar Ophiocomina nigra (Echinodermata: Ophiuroidea) PLoS One 11: e0167533.
Yannas IV (2018) Hesitant steps from the artificial skin to organ regeneration. Regen Biomater 5(4):189–195
Zavalnaya EG, Shamshurina EV, Eliseikina MG (2020) The immunocytochemical identification of PIWI-positive cells during the recovery of a coelomocyte population after evisceration in the holothurian Eupentacta fraudatrix (Djakonov et Baranova, 1958) (Holothuroidea: Dendrochirota). Russ J Mar Biol 46(2):97–104
Becker S, Jarriault S (2016) Natural and induced direct reprogramming: mechanisms, concepts and general principles—from the worm to vertebrates. Curr Opin Genet Dev 40:154–163
Smiley S (1994) Holothuroidea. In: Harrison FW, Chia FS (eds) Microscopic anatomy of invertebrates, vol 14. Echinodermata. Wiley-Liss Inc, New York, pp 401–471
Glauert M, Lewis R (1998) Biological specimen preparation for transmission electron microscopy. In: Glauert, AM (series ed.) Embedding methods. Practical Methods in Electron Microscopy, vol 17: Princeton University Press, pp 147–169
Lai G, Aboobaker A (2018) EvoRegen in animals: Time to uncover deep conservation or convergence of adult stem cell evolution and regenerative processes. Dev Biol 433(2):118–131
Czarkwiani A, Ferrario C, Dylus V, Sugni M, Oliveri P (2016) Skeletal regeneration in the brittle star Amphiura filiformis. Front Zool 13(1):1–17
Dolmatov I, Mashanov V, Zueva O (2007) Derivation of muscles of the Aristotle’s lantern from coelomic epithelia. Cell Tissue Res 327(2):371–384
Wilkie I (2005) Mutable collagenous tissue: overview and biotechnological perspective. Echinodermata 221–250
Acknowledgments
The authors express their gratitude to Dr. Vo Si Tuan (Director of IO VAST) for the opportunity to work at IO VAST and to Mr. Hoang Trung Du for the assistance in collecting the material. Special thanks are due to Dr. E.S. Mekhova for the identification of feather star species.
Funding
This study was supported by the Ministry of Science and Higher Education of the Russian Federation (grant 13.1902.21.0012, contract no. 075-15-2020-796).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
V., K.N., O., K.Y. & Yu., D.I. Regeneration of the digestive system in the crinoid Lamprometra palmata (Mariametridae, Comatulida). Cell Tissue Res 391, 87–109 (2023). https://doi.org/10.1007/s00441-021-03526-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03526-4