Abstract
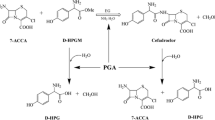
Mass transfer effects were investigated for the synthesis of ampicillin and amoxicillin, at pH 6.5 and 25 °C, catalyzed by penicillin G acylase immobilized on agarose. The influence of external mass transfer was analysed using different stirring rates, ranging form 200 to 800 rpm. Above 400 rpm, the film resistance may be neglected. Intra-particle diffusion limitation was investigated using biocatalysts prepared with different enzyme loads and agarose with different mean pore diameters. When agarose with 6, 8 and 10% of crosslinking were used, for the same enzyme load, substrates and products concentration profiles presented no expressive differences, suggesting pore diameter is not important parameter. An increase on enzyme load showed that when more than 90 IU of enzyme activity were used per mL of support, the system was influenced by intra-particle mass transfer. A reactive-diffusive model was used to estimate effective diffusivities of substrates and products.







Similar content being viewed by others
References
Bianchi D, Golini R, Bortolo R, Cesti P (1996) Immobilization of Penicillin G Acylase on Aminoalkylated Polyacrylic Supports. Enzyme Microb Technol 18:592–596
Arroyo M, De la Mata I, Acebal C, Castillon MP (2003) Biotechnological applications of penicillin acylases: state of the art. Appl Microbiol Biotechnol 60:507–514
Brahim S, Narinesingh D, Guiseppi-Elie A (2002) Kinetics of glucose oxidase immobilized in p(HEMA)-hydrogel microspheres in a packed-bed bioreactor. J Mol Catal B-Enzym 18:69–80
Tischer W, Kasche V (1999) Immobilized enzymes: crystal or carriers? Tibtech 17:326–335
Fernadez-Lafuente RF, Rossel CM, Guisán JM (1995) The use of stabilised penicillin acylase derivatives improves the design of kinetically controlled synthesis. J Mol Catal A-Chem 101:91–97
Gonçalves LRB, Fernadez-Lafuente R, Guisán JM, Giordano RLC (2000) A kinetic study of the synthesis of amoxicillin using penicillin G acylase immobilized on agarose. Appl Biochem Biotechnol 84–86:931–945
Gonçalves LRB, Fernadez-Lafuente R, Guisán JM, Giordano RLC (2002) The role of 6-aminopenicillanic acid on the kinetics of the enzymatic synthesis of amoxicillin catalyzed by penicillin g acylase immobilized on glyoxyl-agarose. Enzyme Microb Technol 31:464–471
Gonçalves LRB, Fernadez-Lafuente R, Guisán JM, Giordano RLC (2003) Inhibitory effects in the side reactions occurring during the enzymatic synthesis of amoxicillin: p-hydroxy-d-phenylglycine methyl ester and amoxicillin hydrolysis. Biotechnol Appl Biochem 38:77–85
Ospina S, Barzana E, Ramırez OT, Lopez-Munguıa A (1996) Effect of pH in the synthesis of ampicillin by penicillin acylase. Enzyme Microb Technol 19:462–469
Kasche V, Haufler U, Zollner R (1984) Kinetic-studies on the mechanism of the Penicillin Amidase-catalyzed synthesis of ampicillin and benzylpenicillin. Hoppe-Seyler’s Physiol Chem 365:1435–1443
Balasingham K, Warburton D, Dunnill P, Lilly MD (1972) Isolation and kinetics of Penicillin Amidase from Escherichia coli. Biochim Biophys Acta 276:250–256
Plaskie A, Roets E, Vanderhaeghe H (1978) Substrate-specificity of Penicillin Acylase of Escherichia coli. J Antibiot 31:783–788
Ferreira ALO, Gonçalves LRB, Giordano RC, Giordano RLC (2000) A simplified kinetic model for the side reactions occurring during the enzymatic synthesis of ampicillin. Braz J Chem Eng 17:835–839
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 11:431–441
Gonçalves LRB, Giordano RLC, Giordano RC (1997) Effects of diffusion on the kinetics of maltose hydrolysis using glucoamylase immobilized on macroporous silica. Braz J Chem Eng 14:341–346
Villadsen JV, Michelsen ML (1978) Solution of differential equation models by polinomial approximation. Prentice Hall, Englewood Cliffs
Petzold LR (1989) DDASSL code, version 1989, Computing and Mathematics Research Division, Lawrence National Laboratory
Giordano RLC, Giordano RC, Cooney CL (2000) A study on intra-particle diffusion in enzymatic reactions: glucose–fructose isomerization. Bioproc Eng 23:159–166
Bittain HG (2005) Solid-state fluorescence of the trihydrate phases of ampicillin and amoxicillin. AAPS Pharm Sci Tech 6:E444–E448
James MNG, Hall D, Hodgkin DC (1968) Crystalline modifications of ampicillin: the trihydrate. Nature 220:168–170
Boles MO, Girven RJ (1976) The structures of ampicillin: a comparison of the anhydrate and trihydrate forms. Acta Crystallogr B32:2279–2284
Boles MO, Girven RJ, Gane PAC (1976) The structures of amoxicillin trihydrate and a comparison with the structures of ampicillin. Acta Crystallogr B34:461–466
Cremasco MA (1998) Fundamentos de Transferência de Massa. Ed. Unicamp, Campinas
Fernández-Sanchez VM (2000) Difusão de aminoácidos e proteínas em partículas de gel de agarose. Universidade Federal de São Carlos
Xiao QG, Tao X, Zhang JP; Chen JF (2006) Hollow silica nanotubes for immobilization of penicillin G acylase enzyme. J Mol Catal B Enzym 42:14–19
Kumar R, Suresh AK, Shankar HS (1996) Kinetics and reaction engineering of penicillin G hydrolysis. J Chem Technol Biot 66:243–250
Acknowledgments
The authors would like to thank Brazilian research-funding agencies FAPESP (State of Sao Paulo), CNPq and CAPES (Federal), Hispanagar S.A. and Antibióticos S.A. for the agarose gel and the enzyme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonçalves, L.R.B., Ferreira, A.L.O., Fernandez-Lafuente, R. et al. Influence of mass transfer limitations on the enzymatic synthesis of β-lactam antibiotics catalyzed by penicillin G acylase immobilized on glioxil-agarose. Bioprocess Biosyst Eng 31, 411–418 (2008). https://doi.org/10.1007/s00449-007-0176-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-007-0176-2