Abstract
Introduction
Infections are a leading cause of morbidity and mortality in patients with multiple myeloma. The epidemiology, risk factors and outcomes of viral respiratory tract infections (vRTI) are not well described in patients with multiple myeloma managed with novel agents, the current standard of care.
Methods
Patients with myeloma from 2009 to 2012 who tested positive on respiratory virus multiplex polymerase chain reaction had clinical, radiological and microbiological records reviewed. The Fourth European Conference on Infections in Leukaemia (ECIL-4) definitions of RTI were applied. Univariate and multivariate regression analysis of risk factors was performed using vRTI as the evaluable outcome.
Results
Of 330 patients, 75 (22.7 %) tested positive for a total of 100 vRTI episodes. All patients received thalidomide, lenalidomide or bortezomib in combination with myeloma therapies (median of three treatment regimens). vRTI occurred most commonly in patients with progressive disease, and receipt of more than three lines of myeloma therapy was associated with an increased risk of vRTI (p < 0.01). Amongst key respiratory pathogens, influenza was associated with the highest hospital admission rate (66.7 %), ICU admission rate (41.6 %) and mortality (33.3 %) whilst RSV was associated with prolonged hospital stay.
Conclusion
Patients with multiple myeloma and advanced disease managed with multiple lines of therapy are at risk for vRTI, and targeted interventions for prevention/treatment are required.
Similar content being viewed by others
Introduction
Multiple myeloma is a clonal plasma cell malignancy, which is increasing in frequency with an annual estimated incidence of 4.8 per 100,000 in the developed world [1]. Infection is the leading cause of mortality in patients with myeloma and increased risk is due to disease and treatment-related factors [2]. The treatment for myeloma has undergone a paradigm shift with the use of newer immunomodulatory drugs (IMiDs) (e.g. thalidomide and lenalidomide) and proteasome inhibitors (PI) (e.g. bortezomib) as standard of care [3]. With this shift, there is evidence of a change in the epidemiology of infections in patients with myeloma towards predominance of viral infections, including respiratory tract infections (RTI) [4].
Whilst the epidemiology, clinical outcomes and risk factors associated with viral RTI (vRTI) are well known in the haematopoietic stem cell transplant (HSCT) setting and supported by appropriate management guidelines, these characteristics have not been reported in myeloma patients managed with novel agents [5, 6]. The objective of this study was to describe the epidemiology, risk factors and outcomes of vRTI in these patients.
Methods
Study population and definitions
Patients were identified retrospectively from chemotherapy drug dispensing and microbiology databases using the following criteria: a confirmed diagnosis of multiple myeloma, received active therapy with IMiD or PI and tested positive for respiratory viral (RV) infection by nucleic acid amplification testing (NAT) at Peter MacCallum Cancer Centre (PMCC) from January 2009 to December 2012. Clinical records of eligible patients were reviewed using a standardized tool to capture the following: patient demographics, myeloma therapy, seasonality of infection, radiological changes, clinical features and outcomes (length of stay (LOS)), requirement for intensive care (ICU) management and all-cause mortality at 30 days).
Influenza vaccination is generally performed by community practitioners and vaccination records were therefore not available for evaluation. For this study, definitions of RTI were consistent with those previously described (Fourth European Conference on Infections in Leukaemia (ECIL-4)) [6]. In brief, an upper respiratory tract infection (URTI) was defined as the detection of RV above the larynx; URTI disease (URTID) was defined as the detection of RV in upper respiratory tract fluid specimens together with symptoms and/or signs; lower RTI disease (LRTID) was defined as clinical symptoms or pulmonary infiltrates together with identification of RVs in respiratory secretions. A confirmed case of respiratory tract infection disease (RTID) was defined in the presence of clinical and laboratory criteria.
Detection of RV more than 1 month after the initial specimen in association with onset of new symptoms was defined as a new infective episode. During the study period, admitted patients with clinical features of upper RTI were routinely managed in a single room with droplet and contact precautions pending the outcome of diagnostic work-up.
During the study period, patients with myeloma received treatment that was consistent with international practices including autologous HSCT (ASCT) for eligible patients or in approved clinical trials. In this study, novel myeloma therapy included thalidomide, lenalidomide and bortezomib used in combination with dexamethasone with or without low-dose cyclophosphamide. For this study, disease periods for a patient with myeloma were defined as follows: Induction period was the time from initial diagnosis until after 4 to 6 cycles of initial treatment, ASCT period included the time from myeloablative chemotherapy (e.g. melphalan) until day 30 post stem cell reinfusion, maintenance was the period with stable paraprotein levels with (or without) myeloma treatment, and progression was any period where rising paraprotein levels necessitated a change in treatment regimen or recommencement of treatment. Chemotherapeutic agents used singly or in combination to obtain or maintain myeloma disease response was defined as a line of therapy.
Diagnostic evaluation
During the study period, the decision to perform and the method of diagnostic evaluation for vRTI was directed by the treating clinician. All samples were analysed by the Victorian Infectious Diseases Reference Laboratory for RV NAT. A respiratory multi-tube multiplex PCR was performed. Targets for the respiratory multiplex PCR included influenza A, B and C viruses; parainfluenza viruses (PV) 1, 2 and 3; respiratory syncytial viruses (RSV) A and B; coronaviruses (OC43, 229E, NL63, HKU and SARS); adenoviruses and picornaviruses (enteroviruses and rhinovirus groups A, B and C). Human metapneumovirus (HMPV) was included for detection in the panel in 2012.
Statistical analysis
Characteristics of patients with vRTI were summarised as proportional outcomes. Categorical variables were compared using the Fisher’s exact test. Univariate and multivariate regression analysis of risk factors (patient age, ASCT, receipt of bortezomib and the number of lines of chemotherapy) was performed using vRTI as the evaluable outcome. Covariates were included in the model if univariate analysis demonstrated p < 0.20. All analyses were performed using Stata (version 9.0, StataCorp Inc., Texas, USA) and p ≤ 0.05 was deemed statistically significant.
Ethics review
The human research ethics committee at our institution approved this retrospective study.
Results
Patient characteristics
Of 330 patients with myeloma managed during the study period, 75 (22.7 %) tested positive for a total of 100 vRTI episodes and an infection rate of 1.2 infections per patient year. Patients with myeloma constituted 19.9 % of 376 patients with malignancies managed at PMCC who tested positive for a RV. Pharyngeal swabs were the most common method of sampling (62 %), followed closely by pooled nasopharyngeal swabs (30 %) and bronchoalveolar lavage (8 %).
Patients with vRTI had a median age of 61 years (interquartile range; 55–66 years) and 56 % were male. Most had IgG myeloma (63 %) and were international staging system stage 1 (63 %) at diagnosis. All patients received thalidomide, lenalidomide or bortezomib in combination myeloma therapies (median of three treatment regimens). The majority of patients 64/75 (85.3 %) had received an ASCT. 23 % of all vRTI episodes were associated with hypogammaglobulinaemia requiring intravenous immunoglobulin replacement. The demographics and myeloma characteristics of the studied population are summarised in Table 1.
Viral respiratory tract infections
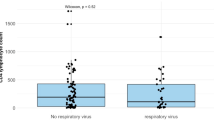
Of 100 episodes, 15 % were upper RTI (URTI), 46 % confirmed URT infectious disease (URTID) and 39 % were confirmed LRTID. Viral RTI occurred most commonly during disease progression 46/100 (46 %). 42 % of episodes required hospital admission with a median LOS of 10 days (interquartile range, 2 to 24 days). Overall, ICU admission rate was 10 % and all-cause mortality rate at 30 days was 6 %.
Of key viral pathogens, influenza vRTI was observed to occur only in patients during maintenance treatment 4/12 (33.3 %) and disease progression 8/12 (66.7 %). The majority of influenza vRTI episodes were LRTID 9/12 (75.0 %) and 8/12 (66.7 %) of episodes were associated with hospital admission with a median LOS of 13 days. Influenza RTI was also associated with the highest ICU admission and all-cause mortality rates of 41.6 and 33.3 %, respectively. Seven of 12 influenza episodes (58.3 %) were treated with oseltamivir.
RSV episodes were associated with hospital admission in 40.0 % of instances and a median LOS of 18 days. Of the RSV episodes, 60.0 % were URTID. RSV RTI was associated with ICU admission in 6.7 % of instances, and no all-cause mortality was recorded at 30 days. No RSV episode was treated with ribavirin, with the exception of one patient with concurrent RSV and influenza A who received both oseltamivir and ribavirin. Frequency of vRTI episodes, myeloma characteristics, infection site and outcomes according to key RV pathogens are summarized in Table 2.
Regression analysis
Univariate regression analysis demonstrated that age ≤65 years (p < 0.01), receipt of ASCT (p = 0.03), bortezomib (p < 0.01) and more than three lines of myeloma therapy (p < 0.01) were associated with vRTI (Table 3). Multivariate logistic regression analysis demonstrated that only receipt of more than three lines of therapy was independently associated with an increased risk of vRTI (p < 0.01).
Discussion
The epidemiology, clinical features and outcomes associated with vRTI are well described in the HSCT setting, in particular, following allogeneic HSCT [5, 6]. To our knowledge, this is the first dedicated study of vRTI in patients with myeloma managed with contemporary treatment including novel myeloma therapies and ASCT. A strength of this study was the fact that both inpatients and outpatients were studied.
We found that vRTI occurred in 22.7 % of patients managed for myeloma and occurred most frequently in patients with progressive disease. In addition, we found receipt of ASCT, bortezomib and more than three lines of therapy were risk factors for vRTI on univariate analysis. There are several possible explanations for these findings. During a vRTI, the innate immune system initiates the host antiviral responses via intracellular signalling pathways and the release of interferon cytokines. These cytokines, including IFN-α, augment host antiviral response and activate the adaptive immune system, which plays a key role in subsequent viral clearance [7]. Corticosteroid treatment for myeloma reduces monocyte counts, function and trafficking to areas of need, whilst IMiDs reduce monocyte activation. Also, bortezomib disrupts NF-Kβ which impacts viral antigen presentation [2]. In addition, patients with myeloma have disease-related immune deficits including defects in cell-mediated and humeral immunity that accumulate with progression of disease [8]. In addition, patients with myeloma are known to have lower levels of specific influenza antibodies compared to healthy controls [9, 10]. These factors may explain our observations that multiple lines of treatment are a risk factor for vRTI and progressive disease being the “at risk” period.
We have demonstrated that vRTI are associated with morbidity in patients with myeloma. Nearly 40 % of vRTI episodes required hospital admission with a median LOS of 10 days. Influenza infections in particular were associated with poor outcomes and increased use of resources. We found over two thirds of influenza vRTI were associated with hospital admission. The majority of influenza episodes (75 %) involved LRTID, which is substantially higher than that reported in previous studies of predominantly allogeneic HSCT patients (30 %) [5, 11]. Influenza episodes were associated with the highest proportion of ICU admission (41.6 %) and all-cause mortality rates (33.3 %) amongst all the RV pathogens. Mortality rates from influenza LRTID in patients with haematological malignancies and HSCT have been reported to be 15–28 % [5, 11]. In fact, patients with advanced myeloma maybe a higher risk group for complications than other patients after HSCT as demonstrated during the recent H1N1 pandemic, where more patients with myeloma required ICU management than patients with other malignancies [12].
This study highlights the need for awareness of RV infections amongst myeloma patients and that a subgroup at high risk for complications can be identified. Preventative measures and treatment should be evaluated in patients with myeloma to reduce the morbidity and mortality associated with influenza infections. The rate of oseltamivir treatment in our cohort may have contributed to the observed mortality, as early treatment of influenza episodes with oseltamivir has been shown to improve outcomes [13]. Seasonal influenza vaccination is well tolerated, effective and results in reduced number of URTI episodes and hospitalisation, albeit from studies undertaken before current treatment regimens were instituted [10, 14]. Current national immunisation and international consensus guidelines recommend annual seasonal influenza vaccination in patients with myeloma, with Australian guidelines recommending two doses, 1 month apart in patients not previously vaccinated [15, 16].
Respiratory syncytial viruses pose a concern to clinicians managing patients with haematological malignancies. We observed similar rates of RSV in our patient cohort compared to other studies [17]. RSV episodes in our population were associated with a modest ICU admission rate (6.7 %) but no mortality. This is in contrast to the expected mortality rates of RSV LRTI of 10–30 % in predominantly allogeneic HSCT patient populations [6]. LOS remained substantial at 18 days. More research is required on the role of antiviral treatments such as ribavirin in this patient population.
Our study has several limitations. This was a retrospective cohort study that used a positive viral result to identify cases. Therefore, as the decision to perform diagnostic evaluation for vRTI was determined by the treating physician, there is likely to be a selection bias favouring symptomatic and unwell patients. In addition, patients with mild infections may present to their local practitioner. Specific indications for ICU admission and attributable cause of death were not determined. The lack of verifiable vaccination history meant we were unable to evaluate the impact of seasonal influenza vaccination on the rates and outcomes of influenza vRTI in our cohort.
Our dedicated study of vRTI in patients with myeloma managed with novel agents and ASCT has identified receipt of multiple lines of therapy (>3) as a risk factor for vRTI with disease progression an at risk period. vRTI, in particular, influenza vRTIs are associated with morbidity and mortality, highlighting the importance of preventative measures (e.g. early treatment, vaccination) and need for further research into treatment in patients with myeloma.
References
UK CR. 2013 November. Cancer stats report - myeloma, Cancer Research UK. Cancer Research United Kingdom <http://www.cancerresearchuk.org/cancer-info/cancerstats/types/myeloma/incidence/%3E. Accessed 29th November 2013
Teh BW, Harrison SJ, Pellegrini M, Thursky KA, Worth LJ, Slavin MA (2014) Changing treatment paradigms for patients with plasma cell myeloma: impact upon immune determinants of infection. Blood Rev 28:75–86
Quach H, Prince HM, Medical Scientific Advisory Group (2012) November. Clinical practice guidelines: multiple myeloma. 1st. Myeloma Foundation of Australia
Teh BW, Thursky KA, Sedunary R, Khot A, Slavin MA, Harrison SJ (2012) Spectrum of infections in myeloma patients treated solely with lenalidomide based regimens and autologous stem cell transplantation. American Society of Microbiology, San Francisco
Nichols WG, Guthrie KA, Corey L, Boeckh M (2004) Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis 39:1300–1306
Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P (2013) Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 56:258–266
Yoo JK, Kim TS, Hufford MM, Braciale TJ (2013) Viral infection of the lung: host response and sequelae. J Allergy Clin Immunol 132:1263–1276, quiz 1277
Pratt G, Goodyear O, Moss P (2007) Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol 138:563–579
Kobold S, Luetkens T, Bartels BM et al (2012) Longitudinal analysis of tetanus- and influenza-specific IgG antibodies in myeloma patients. Clin Dev Immunol 2012:134081
Rapezzi D, Sticchi L, Racchi O, Mangerini R, Ferraris AM, Gaetani GF (2003) Influenza vaccine in chronic lymphoproliferative disorders and multiple myeloma. Eur J Haematol 70:225–230
Chemaly RF, Ghosh S, Bodey GP et al (2006) Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 85:278–287
Tramontana AR, George B, Hurt AC et al (2010) Oseltamivir resistance in adult oncology and hematology patients infected with pandemic (H1N1) 2009 virus, Australia. Emerg Infect Dis 16:1068–1075
Choi SM, Boudreault AA, Xie H, Englund JA, Corey L, Boeckh M (2011) Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood 117:5050–5056
Musto P, Carotenuto M (1997) Vaccination against influenza in multiple myeloma. Br J Haematol 97:505–506
(2013) The Australian immunisation handbook. 10th Edition. In: Ageing AGDoHa (ed). Canberra: DoHA
(2011) Consensus guidelines for Infection Prophylaxis including Vaccination in Multiple Myeloma. Paris, France
Martino R, Porras RP, Rabella N et al (2005) Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant 11:781–796
Acknowledgments
Dr. Benjamin W. Teh is supported by the National Health and Medical Research Council Postgraduate scholarship.
Potential conflicts of interest
SJH has received honoraria and research grant funding from Celgene corporation and Janssen Cilag. All other authors have no conflicts to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teh, B.W., Worth, L.J., Harrison, S.J. et al. Risks and burden of viral respiratory tract infections in patients with multiple myeloma in the era of immunomodulatory drugs and bortezomib: experience at an Australian Cancer Hospital. Support Care Cancer 23, 1901–1906 (2015). https://doi.org/10.1007/s00520-014-2550-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2550-3