Abstract
Purpose
Sarcopenia is associated with reduced survival in cancer. Currently, data on sarcopenia at presentation and muscle loss throughout treatment are unknown in patients receiving chemoradiation therapy (CRT) for non-small cell lung cancer (NSCLC). This study evaluated skeletal muscle changes in NSCLC patients receiving CRT and relationship with survival.
Methods
Secondary analysis of 41 patients with NSCLC treated with CRT assessed for skeletal muscle area and muscle density by computed tomography pre-treatment and 3 months post-treatment. Images at week 4 of treatment were available for 32 (78%) patients. Linear mixed models were applied to determine changes in skeletal muscle over time and related to overall survival using Kaplan-Meier plots.
Results
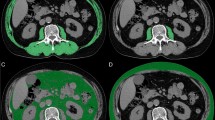
Muscle area and muscle density decreased significantly by week 4 of CRT (− 6.6 cm2, 95% CI − 9.7 to − 3.1, p < 0.001; − 1.3 HU, 95% CI − 1.9 to − 0.64, p < 0.001, respectively), with minimal change between week 4 of CRT and 3 months post-CRT follow-up (− 0.2 cm2, 95% CI − 3.6–3.1, p = 0.91; − 0.27, 95% CI − 0.91–0.36, p = 0.36, respectively). Sarcopenia was present in 25 (61%) and sarcopenic obesity in 6 (14%) of patients prior to CRT, but not associated with poorer survival. Median survival was shorter in patients with low muscle density prior to treatment although not statistically significant (25 months + 8.3 vs 53 months + 13.0, log-rank p = 0.17).
Conclusion
Significant loss of muscle area and muscle density occurs in NSCLC patients early during CRT. A high proportion of patients are sarcopenic prior to CRT; however, this was not significantly associated with poorer survival.

Similar content being viewed by others
References
Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving Capecitabine treatment. Clin Cancer Res 15(8):2920–2926
Baracos V et al (2010) Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr 91:1133S–1137S
Awad S, Tan BH, Cui H, Bhalla A, Fearon KCH, Parsons SL, Catton JA, Lobo DN (2012) Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr 31(1):74–77
Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MAE, den Braver NR, Berkhof J, Langius JAE, Verheul HMW (2016) Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 34(12):1339–1344
Tan BHL, Birdsell LA, Martin L, Baracos VE, Fearon KCH (2009) Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 15(22):6973–6979
Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB (2010) Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 21(8):1594–1598
Antoun S, Lanoy E, Iacovelli R, Albiges-Sauvin L, Loriot Y, Merad-Taoufik M, Fizazi K, di Palma M, Baracos VE, Escudier B (2013) Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 119(18):3377–3384
Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31(12):1539–1547
Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9(7):629–635
Atlan P, Bayar MA, Lanoy E, Besse B, Planchard D, Ramon J, Raynard B, Antoun S (2017) Factors which modulate the rates of skeletal muscle mass loss in non-small cell lung cancer patients: a pilot study. Support Care Cancer 25(11):3365–3373
Australian Cancer Network, Clinical Practice Guidelines for the Prevention, Diagnosis and Management of Lung Cancer, T.C.C. Australia, Editor. 2004, National Health and Medical Research Council
Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr, Engstrom PF, Ezdinli EZ, Horton J, Johnson GJ, Moertel CG, Oken MM, Perlia C, Rosenbaum C, Silverstein MN, Skeel RT, Sponzo RW, Tormey DC (1980) Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am J Med 69(4):491–497
Kiss N, Isenring E, Gough K, Krishnasamy M (2014) The prevalence of weight loss during (chemo)radiotherapy treatment for lung cancer and associated patient- and treatment-related factors. Clin Nutr 33:1074–1080
van der Meij BS, Phernambucq ECJ, Fieten GM, Smit EF, Paul MA, van Leeuwen PAM, Oosterhuis JWA (2011) Nutrition during trimodality treatment in stage III non-small cell lung cancer: not only important for underweight patients. J Thorac Oncol 6(9):1563–1568
Sanders KJC, Hendriks LE, Troost EGC, Bootsma GP, Houben RMA, Schols AMWJ, Dingemans AMC (2016) Early weight loss during chemoradiotherapy has a detrimental impact on outcome in NSCLC. J Thorac Oncol 11(6):873–879
Prado CMM, Heymsfield SB (2014) Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN. J Parenter Enter Nutr 38(8):940–953
Everitt S, Ball D, Hicks RJ, Callahan J, Plumridge N, Trinh J, Herschtal A, Kron T, Mac Manus M (2017) Prospective study of serial imagin comparing flurodeoxyglucose position emission tomography (PET) and fluorothymidine PET during radical chemoradiation for non-small cell lung cancer: reducation of detectable proliferation associated with worse survival. Int J Radiat Oncol Biol Phys 99(4):947–955
Everitt S, Callahan J, Obeid E, Hicks RJ, Mac Manus M, Ball D (2017) Acute radiation oesophagitis associated with 2-deoxy-2-[18F]fluoro-d-glucose uptake on positron emission tomography/CT during chemo-radiation therapy in patients with non-small-cell lung cancer. J Med Imaging Radiat Oncol 61(5):682–688
Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33(5):997–1006
Shen WWZ, Gallagher D, St Onge M, Albu J, Heymsfield S, Heshka S (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single cross-sectional image. J Appl Physiol 97:2333–2338
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495
Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, Antoun S, Baracos VE (2013) Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 98(4):1012–1019
Allison SP (2000) Malnutrition, disease, and outcome. Nutrition 16(7–8):590–593
Ottery F (1996) Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 12(1, Supplement):S15–S19
Cederholm T, Bosaeus I, Barazzoni R, Bauer J, van Gossum A, Klek S, Muscaritoli M, Nyulasi I, Ockenga J, Schneider SM, de van der Schueren MAE, Singer P (2015) Diagnostic criteria for malnutrition – an ESPEN consensus statement. Clin Nutr 34(3):335–340
White JV, Guenter P, Jensen G, Malone A, Schofield M, Academy Malnutrition Work Group, A.S.P.E.N. Malnutrition Task Force, and the A.S.P.E.N. Board of Directors (2012) Consensus statement: academy of nutrition and dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Parenter Enter Nutr 36(3):275–283
Lam VK et al (2017) Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer 104(Supplement C):52–57
Isenring E, Zabel R, Bannister M, Brown T, Findlay M, Kiss N, Loeliger J, Johnstone C, Camilleri B, Davidson W, Hill J, Bauer J (2013) Updated evidence-based practice guidelines for the nutritional management of patients receiving radiation therapy and/or chemotherapy. Nutr Diet 70(4):312–324
Cormie P, Zopf EM, Zhang X, Schmitz KH (2017) The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev 39(1):71–92
Gardner JR, Livingston PM, Fraser SF (2014) Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol Off J Am Soc Clin Oncol 32(4):335–346
Chasen M, Bhargave R (2010) A rehabilitation program for patients with gastrointestinal cancer - a pilot study. Support Care Cancer 18(Supplement 2):S35–S40
Gagnon B, Murphy J, Eades M, Lemoignan J, Jelowicki M, Carney S, Amdouni S, di Dio P, Chasen M, MacDonald N (2013) A prospective evaluation of an interdiscipinary nutrition-rehabilitation program for patients with advanced cancer. Curr Oncol 20(6):310–318
Parmar M, Swanson T, Jagoe RT (2013) Weight changes correlate with alterations in subjective physical function in advanced cancer patients referred to a specialized nutrition and rehabilitation team. Support Care Cancer 21(7):2049–2057
Acknowledgements
The authors would like to thank Jason Callahan and Nick Hardcastle for their technical support and Gavin Abbott for his statistical support
Statement of authorship
NK participated in the design of the study, performed the statistical analysis, interpretation of results and drafted the manuscript. JB participated in data collection, interpretation of results and writing the manuscript. SE participated in the design of the study, interpretation of results and writing the manuscript.
Funding
This work was supported by a National Health and Medical Research Council New Investigator Project Grant (APP1003895) and a Victorian Cancer Agency Tumour Stream Research Grant (both SJE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Approval to conduct the study was granted by the Institutional Research and Ethics committees (ACTRN 12611001283965), all patients provided written, informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kiss, N., Beraldo, J. & Everitt, S. Early Skeletal Muscle Loss in Non-Small Cell Lung Cancer Patients Receiving Chemoradiation and Relationship to Survival. Support Care Cancer 27, 2657–2664 (2019). https://doi.org/10.1007/s00520-018-4563-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4563-9