Abstract
Purpose
The Palliative Care Study Group in conjunction with the Oral Care Study Group of the Multinational Association for Supportive Care in Cancer (MASCC) formed a sub-group to develop evidence-based guidance on the management of common oral problems in patients with advanced cancer.
Methods
This guidance was developed in accordance with the MASCC Guidelines Policy. A search strategy for Medline was developed, and the Cochrane Database of Systematic Reviews and the Cochrane Central Register of Controlled Trials were explored for relevant reviews and trials, respectively. Guidance was categorised by the level of evidence, and “category of guideline” (i.e., “recommendation”, “suggestion” or “no guideline possible”).
Results
Twelve generic suggestions (level of evidence – 5), three problem-specific recommendations and 14 problem-specific suggestions were generated. The generic suggestions relate to oral hygiene measures, assessment of problems, principles of management, re-assessment of problems and the role of dental/oral medicine professionals.
Conclusions
This guidance provides a framework for the management of common oral problems in patients with advanced cancer, although every patient requires individualised management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral symptoms/conditions (“oral problems”) are common in patients with advanced cancer and are the cause of significant morbidity in this group of patients [1]. Hence, it is essential that healthcare professionals involved in the care of patients with advanced cancer have a good understanding about the principles of oral hygiene/care and knowledge of the evidence-based management of common oral problems.
Observational studies suggest that oral problems are not well-managed in patients with advanced cancer [2]. The reasons for the latter are multiple and include inadequate assessment, lack of diagnosis, inappropriate treatment and inadequate re-assessment. Indeed, oral problems are often not assessed by healthcare professionals and importantly often not reported by patients and carers (and represent so-called orphan problems) [3]. Additionally, there are no up-to-date, evidence-based guidelines on the management of oral problems in patients with advanced cancer within the medical and dental literature.
On the basis of the above, the Palliative Care Study Group, in conjunction with the Oral Care Study Group, of the Multinational Association for Supportive Care in Cancer (MASCC) formed a sub-group (“Group”) to develop MASCC/ISOO (International Society of Oral Oncology) evidence-based guidance on the management of common oral problems in patients with advanced cancer. This paper provides an overview of oral problems in patients with advanced cancer, the methodology involved in developing this guidance and the evidence identified in support of the recommendations/suggestions within this guidance.
It should be noted that this guidance purposely does not cover specific cancer treatment–related oral problems, since MASCC/ISOO has already produced a series of evidence-based guidelines on these oral problems (e.g. oral mucositis, salivary gland hypofunction/xerostomia) [4,5,6].
Background
Aetiology
Oral problems may be related to either (a) a direct (“anatomical”) effect of the cancer, e.g. oral discomfort due to intraoral cancer; (b) an indirect (“physiological”) effect of the cancer—see below; (c) an effect of cancer treatment, e.g. salivary gland hypofunction due to head and neck radiotherapy; (d) an effect of a coexisting disease or its treatment or (e) a combination of the above factors [1].
Advanced cancer is associated with a number of sequelae that can indirectly result in oral problems, including physical problems such as fatigue (resulting in difficulty in undertaking oral hygiene), psychological problems such as depression (resulting in lack of motivation/difficulty in undertaking oral hygiene) [7] and relative immunosuppression (resulting in increased risk of oral infections) [8].
Epidemiology
As discussed above, oral symptoms are common in patients with advanced cancer [9,10,11,12,13]. Table 1 shows the results for oral symptoms from a recent study of patients with advanced cancer receiving specialist palliative care in the UK [13]. In this study, 97.5% of patients had at least one oral symptom, and the mean number of oral symptoms was five (range 1–18). Table 2 shows the results for other oral conditions from relevant palliative care studies [9,10,11,12, 14,15,16,17,18,19]. Certain oral problems appear to become more common as the patient’s condition progresses (e.g. xerostomia, oral candidosis) [16, 20].
Clinical features
The mouth is integral to various everyday functions, such as respiration, communication (i.e. verbal, non-verbal), eating and drinking, administration of medication (i.e. oral route, oral transmucosal route) and prevention of infection. Oral problems are a significant direct cause of morbidity in many patients receiving palliative care [13]. Moreover, oral problems can lead to a more generalised deterioration in a patient’s condition [21] and health-related quality of life [12]. Oral problems are also an indirect cause of mortality in some patients receiving palliative care (e.g. oral colonisation/infection leading to respiratory and systemic infections) [8, 22].
Oral problems often co-exist [13], and a sentinel problem can lead to the development of a number of other local and systemic problems (which magnifies the impact of the problem on the patient’s health-related quality of life). For example, salivary gland hypofunction (dry mouth) is associated with oral discomfort, lip discomfort, cracking of lips, taste disturbance (dysgeusia), difficulty chewing (dysmasesia), difficulty swallowing (dysphagia), decreased intake of nutrition, oesophagitis, difficulty speaking (dysphonia), anorexia, poor oral hygiene, halitosis, oral candidosis, dental caries, salivary gland infections (sialadenitis), dental demineralisation (causing dental sensitivity), denture fitting problems, sleep disturbance, embarrassment, anxiety, depression and social isolation [1, 4]. Importantly, the successful management of the sentinel problem may lead to the resolution of some/all of the other local and systemic problems.
Oral problems also impact relationships with family members and others, with some patients avoiding situations that may highlight the problem (e.g. eating with others), and other patients avoiding social interactions altogether [21, 23].
Methods
The aim of the Group was to develop clinically relevant, evidence-based guidance on the management of common oral problems in patients with advanced cancer. Thus, it was agreed that the recommendations could include ones supported by “high” levels of evidence (e.g. systematic reviews), as well as ones supported by “low” levels of evidence (e.g. expert opinion), if the topic was deemed to be clinically relevant and stronger evidence was lacking within the medical and dental literature.
This guidance was developed in accordance with the MASCC Guidelines Policy [6]. The Group adopted the National Cancer Institute (NCI) definition of advanced cancer (i.e. “cancer that has spread to other places in the body and usually cannot be cured or controlled with treatment”) [24], and data was included from studies involving patients receiving disease-oriented/modifying treatment and especially patients receiving symptom-oriented treatment (i.e. palliative care). Other terms utilised within this review include “end-of-life” (i.e. the last year of life) [25] and “terminal phase” (i.e. the last days to weeks of life) [26].
A search strategy for Medline was developed (Appendix 1), and the Cochrane Database of Systematic Reviews, the Cochrane Oral Health Group’s inventory of systematic reviews and the Cochrane Central Register of Controlled Trials (CENTRAL) were explored for relevant systematic reviews/randomised clinical trials, respectively [27]. The review of the published literature was restricted to papers written in English and to papers relating to adult (> 18 years) humans.
All abstracts identified by the search of Medline were downloaded into a reference management software package (EndNote X9, Clarivate™). These abstracts were independently assessed for relevance by two authors (JJ, AD), and if one author deemed the abstract relevant, then the full text of the article was obtained. The criteria utilised were (a) relevant population; (b) relevant/common clinical problem and (c) relevant/non-specialist treatment intervention. The full text articles were independently assessed for inclusion by the same two authors (JJ, AD). The criteria utilised were (a) relevance to guidance and (b) quality of contents. Moreover, these two authors (JJ, AD) were involved in assessing the systematic reviews in the Cochrane Database of Systematic Reviews/Cochrane Oral Health Group’s inventory of systematic reviews. All of the authors were involved in assessing the randomised controlled trials in CENTRAL.
Generic guidance was often based on standard (evidence-based) dental and oral medicine practice, but specific guidance was invariably restricted to data arising from research studies involving patients with advanced cancer. Guidance was characterised by a level of evidence (i.e. I–V) and a “category of guideline” (i.e. “recommendation”, “suggestion” or “no guideline possible”) (Appendix 2) [6]. All of the authors agree with the final content of the guidance, including the levels of evidence and the categories of guideline.
Results
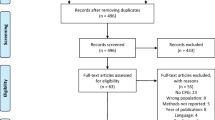
The Medline search (original search—24th August 2020; updated searches—29th March 2021 and 5th January 2022) produced 9802 references, and 197 full text articles were retrieved/reviewed. The Cochrane Central Register of Controlled Trials search (search dates same as Medline) produced 6241 references, of which 27 were deemed relevant, including 15 unique studies (c.f. Medline). The analogous searches of the Cochrane Database of Systematic Reviews (search limited to “cancer”) identified one review, which was not identified in the Medline search; the analogous searches of the Cochrane Oral Health Group inventory of systematic reviews (search not limited to cancer) identified 11 potentially relevant reviews, five of which were not identified in the Medline search.
On the basis of the available evidence, the Group generated 12 generic “suggestions”, as well as three problem-specific “recommendations” and 14 problem-specific “suggestions” (see below).
Generic suggestions
The generic suggestions are summarised in Box 1. The problem-specific recommendations/suggestions are included in the main text. Additionally, the Group recommends that pharmacological interventions are used in accordance with their Summary of Product Characteristics, i.e. that prescribers follow the prescribing guidance (e.g. dose, schedule) and take note of relevant contraindications, cautions, interactions and adverse effects.
Box 1 Generic suggestions about oral care in patients with advanced cancer.
1—All patients with advanced cancer should be regularly assessed for oral problems (Category of guideline – suggestion; Level of evidence—V) 2—All patients need a regular oral hygiene regimen, and dependent patients need appropriate support with oral hygiene (Category of guideline – suggestion; Level of evidence—V) 3—The management of oral problems should primarily involve treatment of the underlying cause (with appropriate symptom control) (Category of guideline – suggestion; Level of evidence—V) 4—The management of oral problems should be individualised (Category of guideline – suggestion; Level of evidence—V) 5—The management of oral problems should be evidence-based and/or based upon established principles from dentistry/oral medicine (Category of guideline – suggestion; Level of evidence—V) 6—Relevant treatments/interventions should be available in all settings wherever possible (Category of guideline – suggestion; Level of evidence—V) 7—All patients with oral problems should be regularly reassessed (Category of guideline – suggestion; Level of evidence—V) 8—Patients with resistant oral problems should be referred to a specialist for further management (Category of guideline – suggestion; Level of evidence—V) 9—Dental professionals should be members of the extended oncology and palliative care multidisciplinary teams (Category of guideline – suggestion; Level of evidence—V) 10—Oral care in the terminal phase should focus on patient comfort (Category of guideline – suggestion; Level of evidence—V) 11—Oral care in the terminal phase is not a substitute for clinically assisted hydration (Category of guideline – suggestion; Level of evidence—V) 12—Oral care should be an integral component of medical and nursing curricula (undergraduate, postgraduate) (Category of guideline – suggestion; Level of evidence—V) |
All patients with advanced cancer should be regularly assessed for oral problems (Category of guideline – suggestion; Level of evidence—V)
As discussed, oral problems are practically universal in patients with advanced cancer, and therefore all such patients should be regularly assessed for such problems [28]. The objectives of assessment are to determine (a) the presence of oral problems; (b) the impact of these problems; (c) the cause of these problems; (d) factors that may affect the choice of intervention (i.e. patient-related factors, e.g. co-morbidities; disease-related factors, e.g. prognosis) and (e) patient preference for interventions. Inadequate assessment may result in initiation of inappropriate or contra-indicated interventions.
Assessment primarily involves taking a focussed history and performing a systematic oral examination: in some cases, clinical evaluation may require confirmatory diagnostic investigations (e.g. microbial cultures, dental images). Older generic oral assessment tools are invariably not appropriate/not validated in patients with advanced cancer (e.g. Oral Assessment Guide) [29]. However, an oral symptom assessment tool has been developed/validated in this group of patients (Oral Symptom Assessment Scale) [13], and newer generic tools may also have a role in patients with advanced cancer (e.g. EORTC QLQ-OH15) [30].
Patients should be questioned about common oral symptoms (Table 1), since they may not report such symptoms spontaneously (even when they cause distress) [3]. Importantly, patients should be examined for the presence of oral conditions, even in the absence of oral symptoms. A good direct light source is essential to facilitate adequate visualisation. Gloves must be worn during the examination, and a gloved finger (or a “tongue depressor”/similar implement) used to retract tissues to again facilitate adequate visualisation [31]. Patients with dentures should have these removed during the examination (as they may obscure pathology). Moreover, it is important that denture stability is assessed, and that the denture is examined for any related problems (e.g. poor hygiene, rough edges).
The assessment of oral problems should be responsibility of all healthcare professionals involved in the care of patients with advanced cancer (and not delegated, or assumed to be delegated, to specific groups of healthcare professionals). However, patients with complex oral problems and those relating to the dentition, periodontal tissues and dental prostheses should be referred in a timely manner to relevant dental professionals for specialist treatment.
All patients need a regular oral hygiene regimen, and dependent patients need appropriate support with oral hygiene (Category of guideline – suggestion; Level of evidence—V)
Good oral hygiene reduces the risk of developing many oral problems in patients with advanced cancer (e.g. halitosis, oral infections). The principles of good oral hygiene are outlined in Box 2 [32]. Care must be taken when assisting patients with their oral hygiene, and gloves and a face mask may be indicated when undertaking oral care (infection control measure). The principles of denture care are outlined in Box 3 [32, 33]. Patients with significant oral hygiene problems, particularly those with “complex” dental restorations, or significant dental/oral conditions, should be referred to relevant dental professionals for specialist treatment.
Box 2 Daily oral hygiene measures [32]
▪Cleaning of the teeth should be done twice daily using a small headed, soft texture, nylon filament toothbrush and a fluoridated toothpaste. Toothpaste should contain at least 1000 ppm fluoride, but patients with salivary gland hypofunction (especially patients with radiation-induced salivary gland hypofunction) require a toothpaste with 5000 ppm fluoride as well as additional fluoride supplements (e.g., rinses, gels) ▪Alternative toothbrushes/adapted toothbrushes may be needed in some patients (e.g., patients with oral discomfort, patients with disabilities) ▪Toothbrushes should be replaced every 3 months, or when the filaments become misshapen, or when there has been an oral infection ▪Alternative toothpastes/water alone may be needed in some patients (e.g., patients with oral discomfort, patients with dysphagia). A variety of toothpastes are available, including non-foaming (“SLS-free”) options, mint-free options and toothpastes for “sensitive teeth” ▪Chemical plaque control should be utilised in patients unable to clean their teeth (e.g., chlorhexidine mouthwash). Chlorhexidine will prevent the development of plaque, but will not remove plaque in situ (which needs to be removed mechanically). Chlorhexidine is used twice daily ▪Interdental cleaning should be done once daily using dental floss, dental tape or interdental brushes ▪Cleaning of the oral mucosa should be done after every meal and involves either rinsing the mouth with water, or using a moistened gauze (if the patient is unable to rinse their mouth with water) |
Box 3 Daily denture hygiene measures [32, 33]
▪Dentures should be removed overnight [32, 33] ▪Dentures should be cleaned outside the mouth, and over a bowl/sink of water (to prevent damage if dropped) [32] ▪Cleaning involves once daily (usually at night) mechanical cleaning using a denture brush or a toothbrush, and a non-abrasive denture cleanser (but not toothpaste) [33]. Alternative options include using a nailbrush, and soap and water [32]. Dentures should be rinsed after meals [32] ▪Cleaning also involves once daily (usually overnight) biochemical cleaning using a commercial denture-cleansing solution [33]. Alternative options include using dilute sodium hypochlorite (non-metallic dentures), or chlorhexidine (all dentures) [32]. The latter should be considered in patients with oral infections. Dentures should be rinsed after cleaning [32] ▪It is important to also clean any remaining teeth, periodontium and the oral mucosa (see Box 2) [32, 33] |
The management of oral problems should primarily involve treatment of the underlying cause (with appropriate symptom control) (Category of guideline – suggestion; Level of evidence—V)
Many oral problems have potentially treatable underlying causes. In such cases, the optimal management is treatment of the underlying cause, although patients may also require symptomatic treatment. If the underlying cause is not treated, it is likely that the oral problem will persist (and even worsen). Moreover, there is an increased risk of developing complications. For example, halitosis may be the result of periodontal disease [34], and non-specific measures to manage halitosis may not resolve this problem (see below). Additionally, uncontrolled periodontal disease may progress to cause important complications such as local infection (abscess), systemic infection (pneumonia), and/or loss of dentition.
The management of oral problems should be individualised (Category of guideline – suggestion; Level of evidence—V)
As with other problems in this group of patients, the management of oral problems should be individualised. Patients should receive the “optimal” management, which invariably involves treatment of the underlying cause (point 3). However, interventions may need to be amended as a result of the patient’s performance status, their co-morbidities and especially their personal preferences. For example, the optimal management of poorly fitting dentures is to replace the relevant denture. However, relining the denture is an alternative strategy and can be performed at the bedside/in the patient’s home [35].
The management of oral problems should be evidence-based and/or based upon established principles from dentistry/oral medicine (Category of guideline – suggestion; Level of evidence—V)
Unfortunately, oral care within clinical practice (palliative care) is often based on “anecdotal remedies” [36], with little regard for the established principles of oral care developed within dentistry/oral medicine. Thus, many recommended interventions are relatively ineffective, or completely inappropriate, for their stated purpose(s) (Table 3) [2].
Relevant treatments/interventions should be available in all settings wherever possible (Category of guideline – suggestion; Level of evidence—V)
All patients should have access to the optimal treatment for their oral problems, although treatment needs to be individualised according to the patient’s condition, their care setting and their ability to attend other care settings (e.g. dental surgery, hospital). Nevertheless, most common interventions can be provided in any care settings, and some dental procedures can be performed at the bedside/in the patient’s home (“domiciliary dental care”) [35].
All patients with oral problems should be regularly reassessed (Category of guideline – suggestion; Level of evidence—V)
All patients with oral problems need to be reassessed in a timely manner (after initiation of treatment), and regularly going forward [28]. The objectives of re-assessment are to determine (a) changes in the clinical condition; (b) adherence with any treatment; (c) the effectiveness of the treatment and (d) the tolerability of the treatment. Inadequate reassessment may result in continuation of ineffective interventions and so persistence or progression of the oral problem.
Patients with recurrent oral problems often have an ongoing underlying cause(s). For example, oral candidosis is often associated with xerostomia/salivary gland hypofunction in patients with advanced cancer [16, 17], and although the clinical infection may respond to a course of antifungal medication, it invariably reoccurs after a short period of time (if the salivary gland hypofunction is not adequately managed—see the “Dry mouth (xerostomia; salivary gland hypofunction)” section).
Patients with resistant oral problems should be referred to a specialist for further management (Category of guideline – suggestion; Level of evidence—V)
Patients with ongoing oral problems should be referred to an appropriate specialist for further investigation and management (e.g. experienced dental professional, special care dentist, oral medicine professional), especially when the problem is unexplained, is unresponsive (to primary treatment) and/or worsens over time.
Dental professionals should be members of the extended oncology and palliative care multidisciplinary teams (Category of guideline – suggestion; Level of evidence—V)
Dental professionals are essential members of the oncology multidisciplinary team [44], particularly in terms of preventing and managing the oral complications of anticancer treatment. Similarly, dental professionals are essential members of the extended palliative care multidisciplinary team [45], although there is limited information on their actual roles within these teams [46]. Clearly, an important role is to provide specialist management of complex oral problems and those relating to the dentition, periodontal tissues and dental prostheses [46]. However, an equally important role is to provide training to non-specialist healthcare professionals on maintaining oral hygiene and managing oral problems.
Oral care in the terminal phase should focus on patient comfort (Category of guideline – suggestion; Level of evidence—V)
Oral care often takes centre stage during the terminal phase (i.e. last days/weeks of life), although the objective outcomes of regular end-of-life “mouth care” regimens are frequently disappointing [47]. The primary aim of oral care should be to maintain oral comfort, rather than to maintain pristine oral hygiene [1]. Indeed, oral hygiene measures often need amending during this period due to patient-related factors. Oral care should be paused and/or discontinued, if there is any indication that it causes distress or discomfort to the patient [1].
Oral care is often delegated to families during the terminal phase. Some family members welcome this task, whilst others find it difficult and/or distressing. It is important, if appropriate, that families are given the opportunity to provide oral care [1]. Equally, it is important that families are not coerced into providing oral care. Furthermore, healthcare professionals must provide adequate instructions and ongoing support and supervision [1]: the former includes the goal of oral care, i.e. to maintain oral comfort (see above re pausing/discontinuing oral care).
Oral care in the terminal phase is not a substitute for clinically assisted hydration (Category of guideline – suggestion; Level of evidence—V)
Dry mouth is a common problem in patients at the end-of-life, and small amounts of water (“mouth care”) may temporarily relieve this symptom. Thirst is a less common problem in this group of patients, and again small amounts of water may again temporarily relieve this symptom [48]. However, thirst often reflects dehydration, and small amounts of water will not reverse dehydration (or equally maintain hydration). Hence, oral care should be considered a comfort measure, and not a substitute for clinically assisted hydration (in applicable patients).
Oral care should be an integral component of all medical and nursing curricula (undergraduate and postgraduate) ( Category of guideline—suggestion; Level of evidence - V)
Medical and nursing curricula generally include minimal education on oral care and/or oral problems [49]. However, given the prevalence of oral problems, and their relevance of oral problems to general health, these topics should be an integral component of all medical and nursing curricula worldwide. Moreover, dental professionals should be involved in curricula development, and particularly the development of educational resources (to ensure that the content is appropriate).
The evidence base for the above generic suggestions is limited, and the evidence base for the following specific suggestions is equally limited. Hence, further research is required in this group of patients. Research in patients with advanced cancer is challenging but not impossible (as researchers have consistently demonstrated) [50,51,52,53]. In the meantime, oral care in patients with advanced cancer can be based on evidence derived from other groups of patients (and not on clinical anecdotes) [36].
Specific recommendations
Dry mouth (xerostomia; salivary gland hypofunction)
Terminology
Xerostomia refers to a subjective sensation of dryness of the mouth, whilst salivary gland hypofunction refers to an objective decrease in salivary gland secretion(s).
Aetiology
There are many potential causes for a dry mouth in patients with advanced cancer [1]. However, the main cause relates to the adverse effects of medication used within general medicine and oncology/palliative care [54].
Management
Management should include treatment of the underlying cause (if possible), e.g. discontinuation of offending medication (Category of guideline—suggestion: Level of evidence—V). Symptomatic management involves both non-pharmacological and pharmacological interventions [55].
Non-pharmacological interventions include water, artificial salivas (Category of guideline—recommendation: Level of evidence—II, i.e. mucin-based artificial saliva) [50,51,52], chewing gum (Category of guideline—recommendation: Level of evidence—II) [52] and acupuncture (No guideline possible) [56, 57]. Artificial salivas tend to have a longer duration of action than water [1], and a number of commercial products are available, which differ in formulation (e.g. spray, gel), pH (e.g. acidic, neutral), lubricant (e.g. mucin, carboxymethylcellulose) and additional ingredients (e.g. flavourings, fluoride). Ideally, an artificial saliva should have a neutral pH (to limit demineralisation of the teeth) and contain fluoride (to enhance remineralisation of the teeth).
Pharmacological interventions include pilocarpine (Category of guideline—recommendation: Level of evidence—II) [52] and related products (e.g. bethanechol, cevimeline). (Currently, there is no evidence to support the use of intraoral pilocarpine eyedrops in this condition [58]).
Saliva stimulants (e.g. sugar-free chewing gum, pilocarpine) are generally preferred to saliva substitutes (e.g. water, artificial salivas) when the salivary glands can be stimulated, since they are more effective in relieving xerostomia, and they should improve the other problems associated with salivary gland hypofunction (see above) [1]. It should be noted that the evidence on managing xerostomia/salivary gland hypofunction per se is of limited quality [59, 60].
Taste disturbance
Terminology
Patients with advanced cancer report a variety of taste disturbances, which include ageusia (an absence of taste sensation), hypogeusia (a decrease in taste sensation), hypergeusia (an increase in taste sensation) and dysgeusia (a distortion of normal taste sensation) [61, 62].
Aetiology
There are several potential causes of new-onset taste disturbance in patients with advanced cancer, including salivary gland hypofunction, poor oral hygiene, medication [63] and nutritional deficiencies (e.g. zinc). Moreover, taste disturbance may be an ongoing complication of the cancer and/or the oncological treatment (e.g. systemic chemotherapy, head and neck radiotherapy) [64].
Management
Management should include treatment of the underlying cause (if possible), e.g. treatment of salivary gland hypofunction (Category of guideline—suggestion: Level of evidence—IV) [65]. Symptomatic management primarily involves non-pharmacological interventions.
Patients with taste disturbance should be reviewed by a dietitian, and a personalised nutritional plan developed (Category of guideline—suggestion: Level of evidence—V). Strategies that may be useful include utilisation of foods that taste “good”; avoidance of foods that taste “bad”; enhancing the taste of the food using salt, sugar and other flavourings; moistening the food and addressing the presentation, smell, consistency and temperature of the food, i.e. paying attention to other aspects of flavour (Category of guideline—suggestion: Level of evidence—V) [66, 67].
Pharmacological interventions reported to be effective in different cohorts of patients with cancer, include zinc supplements (no guideline possible) [68], dronabinol (Category of guideline—suggestion: Level of evidence—II, i.e. pilot study) [69], megestrol acetate (no guideline possible) [70] and clonazepam (no guideline possible) [65].
Oral discomfort/pain
Aetiology
There are several potential causes of new onset oral discomfort/pain in patients with advanced cancer, including salivary gland hypofunction [54], mucosal conditions (i.e. infection/inflammation/ulceration) [12, 71], dental-related problems, denture-related problems [71], neurological conditions and pain referred from adjacent structures.
Management
Management should include treatment of the underlying cause (if possible), e.g. relining, adjusting, or replacing poorly fitting dentures (Category of guideline—suggestion: Level of evidence—V). Symptomatic management primarily involves pharmacological interventions, which may include both topical agents and systemic analgesics. The optimal analgesic regimen depends on the aetiology of the pain (cancer-related, coexisting condition), the pathophysiology of the pain (nociceptive, neuropathic) and a variety of patient-related factors (e.g. co-morbidities, personal preference) (Category of guideline—suggestion: Level of evidence—V) [72].
Bland rinses (e.g. normal saline) can often provide some relief for patients with oral ulceration, including patients with oral mucositis. Similarly, “coating” agents can provide some relief for patients with oral ulceration, particularly in patients with pain on eating/drinking (“contact”/incident pain). However, many patients with oral ulceration have associated inflammation, and so merely covering the ulcer(s) does not relieve the discomfort. Topical analgesics can be highly effective in some cases, and options include benzydamine hydrochloride solution/spray (anti-inflammatory), oral morphine solution (opioid) and doxepin solution (antidepressant) [73]. Topical analgesics are less likely to cause systemic adverse effects, and so are often considered as first line treatments. Local anaesthetics have a limited role, and care must be taken to prevent inadvertent aspiration. Equally, topical steroids have a limited role (e.g. recurrent aphthous stomatitis), and care must be taken to exclude oral problems that might worsen with steroids (e.g. oral infections).
Systemic analgesics can also be effective, but there are limited reasons for chronic administration (due to concerns about adverse effects).
Interventional techniques are another option in highly select cohorts of patients (e.g. patients with intraoral tumours).
Halitosis
Terminology
Patients with genuine halitosis (i.e. objective evidence of malodour) can be subclassified into those with physiological halitosis (i.e. no evidence of underlying condition) and pathological halitosis (i.e. evidence of underlying condition).
Aetiology
Physiological halitosis is the most common type: the cause of physiological halitosis is the bacterial putrefaction of food debris, epithelial cells, blood cells and saliva within the oral cavity (and particularly on the posterior part of the dorsum of the tongue) and poor/limited oral hygiene. The cause of pathological halitosis may be an infection, inflammation or malignancy. In ~ 90% such cases, the source of the malodour is the oral cavity, but other potential sources include the upper respiratory tract (nose, sinuses), the lower respiratory tract, the gastrointestinal tract or a systemic metabolic problem.
Management
Management depends on the type of halitosis: in patients with physiological halitosis, it primarily involves oral hygiene measures, whilst in patients with pathological halitosis, it primarily involves treatment of the underlying cause (if possible), e.g. antibiotics for infections (Category of guideline—suggestion: Level of evidence—V).
The management of physiological halitosis includes [74] (Category of guideline—suggestion: Level of evidence—V) (a) generic oral hygiene measures (see above); (b) avoidance of odorous foodstuffs (e.g. garlic, onions); (c) alcohol cessation; (d) smoking cessation; (e) measures to reduce bacterial numbers (e.g. tongue cleaning, chlorhexidine—mouthwash); (f) measures to reduce bacterial substrate (e.g. tongue cleaning, “professional” dental/periodontal cleaning) and (g) measures to convert offensive volatile sulphur compounds to inoffensive non-volatile compounds (e.g. zinc salts—toothpaste, mouthwash; sodium bicarbonate/baking soda – toothpaste). Many oral care products are available to manage halitosis, and those with specific properties (see above) should be prescribed in preference to those with simply “masking”/cosmetic properties (Category of guideline—suggestion: Level of evidence—V). It should be noted that the evidence on managing halitosis in general is of limited quality [75].
Oral candidosis
Aetiology
Candida species are opportunistic microorganisms, and so oral candidosis usually occurs as a result of changes in host systemic and/or intraoral factors [76]. In patients with advanced cancer, oral candidosis has been found to be specifically associated with poor performance status, salivary gland hypofunction, presence of denture and use of systemic steroids [16, 17]. However, other factors may also have a role in the development of oral candidosis in this group of patients (e.g. immunosuppression, systemic antibiotics, mucosal damage) [77].
Management
The management of confirmed oral candidosis primarily involves the use of antifungal medication (i.e. polyenes, azoles). However, antifungal medication (especially azoles) should preferably be reserved for patients with laboratory-confirmed oral candidosis (Category of guideline—suggestion: Level of evidence—V), since other oral conditions can have similar features, and antifungal resistance is reported to be common in this group of patients [78,79,80]. Importantly, antifungal medication should be combined with treatment of predisposing factors (see above) (Category of guideline—suggestion: Level of evidence—V), since many patients will recur after the course of antifungal medication is completed (due to persistence of predisposing factors).
A variety of topical and systemic antifungal medications are available for treating oral candidosis. The choice of treatment depends on a number of factors [1] (Category of guideline—suggestion: Level of evidence—V): (a) extent of disease – topical agents are appropriate for treating localised disease, whilst systemic agents are more appropriate for treating multifocal/generalised disease; (b) immunocompetence – systemic agents are more appropriate for treating immunosuppressed patients; (c) drug resistance – resistance to the polyenes is uncommon, although resistance to the azoles is relatively common; (d) concomitant disease – azoles have a number of relative/absolute contraindications; (e) concomitant medication – systemic azoles have a number of drug interactions; (f) patient preference; (g) ease of use – topical agents are more difficult to use, and efficacy is dependent on correct usage (i.e. contact with lesions) and (g) patient adherence. (Currently, there is no robust evidence to support the use of single doses of fluconazole in this condition [81] (no guideline possible)).
Other options for patients with recurrent/resistant oral candidosis, or patients with difficulties in using conventional antifungal medication, include chlorhexidine (no guideline possible) [82] and tea tree oil (no guideline possible) [83].
It should be noted that the successful management of denture-related stomatitis (with/without angular cheilitis) depends on a combination of antifungal drug treatment, and disinfection of the denture (see Box 3) (Category of guideline—suggestion: Level of evidence—V). When the patient wears a denture, the denture should be treated as well to prevent reinfection of the oral cavity, e.g. by immersing it in a chlorhexidine mouth rinse.
Conclusion
This guidance provides an evidence-based framework for the management of common oral problems in patients with advanced cancer, although every patient requires individualised management (based upon a thorough assessment). Many of the recommendations are based on low levels of evidence, and/or evidence extrapolated from other groups of patients with cancer. However, patients with advanced cancer are in many ways a unique group, and so further research is warranted in this specific group in order to improve their oral care (and quality of life).
Data availability
Not applicable.
Code availability
Not applicable.
References
Davies A (2021) Oral care. In: Cherny N, Fallon M, Kaasa S, Portenoy RK, Currow DC (eds) Oxford textbook of palliative medicine, 6th edn. Oxford: Oxford University Press, Oxford, pp 656–669.
Kvalheim SF, Strand GV, Husebø BS, Marthinussen MC (2016) End-of-life palliative oral care in Norwegian health institutions. Exploratory Study Gerodontol 33:522–529
Shorthose K, Davies A (2003) Symptom prevalence in palliative care. Palliat Med 17:723–724
Mercadante V, Jensen SB, Smith DK, Bohlke K, Bauman J, Brennan MT et al (2021) Salivary gland hypofunction and/or xerostomia induced by nonsurgical cancer therapies: ISOO/MASCC/ASCO guideline. J Clin Oncol 39:2825–2843
Elad S, Cheng KKF, Lalla RV, Yarom N, Hong C, Logan RM et al (2020) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 126:4423–4431
Multinational association for supportive care in cancer website: https://www.mascc.org/ Accessed 24th December 2021
Friedlander AH, Mahler ME (2001) Major depressive disorder. Psychopathology, medical management and dental implications. J Am Dent Assoc 132:629–638
Lerman MA, Laudenbach J, Marty FM, Baden LR, Treister NS (2008) Management of oral infections in cancer patients. Dent Clin North Am 52:129–153
Jobbins J, Bagg J, Finlay IG, Addy M, Newcombe RG (1992) Oral and dental disease in terminally ill cancer patients. BMJ 304:1612
Alt-Epping B, Nejad RK, Jung K, Gross U, Nauck F (2012) Symptoms of the oral cavity and their association with local microbiological and clinical findings - a prospective survey in palliative care. Support Care Cancer 20:531–537
Wilberg P, Hjermstad MJ, Ottesen S, Herlofson BB (2012) Oral health is an important issue in end-of-life cancer care. Support Care Cancer 20:3115–3122
Fischer DJ, Epstein JB, Yao Y, Wilkie DJ (2014) Oral health conditions affect functional and social activities of terminally ill cancer patients. Support Care Cancer 22:803–810
Davies A, Buchanan A, Todd J, Gregory A, Batsari KM (2021) Oral symptoms in patients with advanced cancer: an observational study using a novel oral symptom assessment scale. Support Care Cancer 29:4349–4356
Chaushu G, Bercovici M, Dori S, Waller A, Taicher S, Kronenberg J et al (2000) Salivary flow and its relation with oral symptoms in terminally ill patients. Cancer 88:984–987
Davies AN, Broadley K, Beighton D (2002) Salivary gland hypofunction in patients with advanced cancer. Oral Oncol 38:680–685
Davies AN, Brailsford SR, Beighton D (2006) Oral candidosis in patients with advanced cancer. Oral Oncol 42:698–702
Davies AN, Brailsford SR, Beighton D, Shorthose K, Stevens VC (2008) Oral candidosis in community-based patients with advanced cancer. J Pain Symptom Manage 35:508–514
Aldred MJ, Addy M, Bagg J, Finlay I (1991) Oral health in the terminally ill: a cross-sectional pilot survey. Spec Care Dentist 11:59–62
Gordon SR, Berkey DB, Call RL (1985) Dental need among hospice patients in Colorado: a pilot study. Gerodontics 1:125–129
Matsuo K, Watanabe R, Kanamori D, Nakagawa K, Fujii W, Urasaki Y et al (2016) Associations between oral complications and days to death in palliative care patients. Support Care Cancer 24:157–161
Rydholm M, Strang P (2002) Physical and psychosocial impact of xerostomia in palliative cancer care: a qualitative interview study. Int J Palliat Nurs 8:318–323
Mandel ID (2004) Oral infections: impact on human health, well-being, and health-care costs. Compend Contin Educ Dent 25:881–882,884,888–890.
Ezenwa MO, Fischer DJ, Epstein J, Johnson J, Yao Y, Wilkie DJ (2016) Caregivers’ perspectives on oral health problems of end-of-life cancer patients. Support Care Cancer 24:4769–4777
National Cancer Institute Dictionary of Cancer Terms: https://www.cancer.gov/publications/dictionaries/cancer-terms/ Accessed 24 December 2021
General Medical Council (2010) Treatment and care towards the end of life: good practice in decision making: https://www.gmc-uk.org/-/media/documents/treatment-and-care-towards-the-end-of-life---english-1015_pdf-48902105.pdf?la=en&hash=41EF651C76FDBEC141FB674C08261661BDEFD004. Accessed 24 December 2021
Lacey J (2015) Management of the actively dying patient. In: Cherny NI, Fallon MT, Kaasa S, Portenoy RK, Currow DC (eds) Oxford textbook of palliative medicine, 5th edn. Oxford University Press, Oxford, pp 1125–1133
Cochrane Library website: https://www.cochranelibrary.com/ Accessed 24 December 2021
British Society for Disability and Oral Health Guidelines for the development of local standards of oral health care for dependent, dysphagic, critically ill and terminally ill patients (2000): http://www.bsdh.org/documents/depend.pdf Accessed 24 December 2021
Eilers J, Berger AM, Petersen MC (1988) Development, testing, and application of the oral assessment guide. Oncol Nurs Forum 15:325–330
Hjermstad MJ, Bergenmar M, Bjordal K, Fisher SE, Hofmeister D, Montel S et al (2016) International field testing of the psychometric properties of an EORTC quality of life module for oral health: the EORTC QLQ-OH15. Support Care Cancer 24:3915–3924
Davies A (2005) Oral assessment. In: Davies A, Finlay I (eds) Oral care in advanced disease. Oxford University Press, Oxford, pp 7–19
Sweeney P (2005) Oral hygiene. Davies A, Finlay I (eds) Oral care in advanced disease. Oxford University Press, Oxford, pp 21–35.
Oral Health Foundation. White paper on optimal care and maintenance of full dentures for oral and general health (2018): https://www.dentalhealth.org/denturecareguidelines Accessed 24 December 2021
De Geest S, Laleman I, Teughels W, Dekeyser C (2000) Quirynen M (2016) Periodontal diseases as a source of halitosis: a review of the evidence and treatment approaches for dentists and dental hygienists. Periodontol 71:213–227
Walls A (2005) Domiciliary dental care. In: Davies A, Finlay I (eds) Oral care in advanced disease. Oxford University Press, Oxford, pp 37–45
Lucas VS, Roberts GJ (1998) Mouth care and skin care in palliative medicine. Chlorhexidine mouth washes are important in mouth care. BMJ 316:1246
Joint Formulary Committee. British National Formulary (2021): http://www.medicinescomplete.com Accessed 24 December 2021
Adkinson L, Hussain J, Daniel S, Oxberry S (2014) Oral health is an important issue in end-of-life care, December 2012. Support Care Cancer 22:293–294
National Institute for Health and Care Excellence. Clinical Knowledge Summaries: Palliative Care - Oral (2021): https://cks.nice.org.uk/topics/palliative-care-oral/ Accessed 24 December 2021
Blackman JG (1890) Pilocarpin in dryness of the tongue. BMJ 1(1536):1366–1367
Poland JM, Dugan M, Parashos P, Irick N, Dugan W, Tracey M (1987) Comparing Moi-Stir to lemon-glycerin swabs. Am J Nurs 87:422–424
Anonymous (2019) Gastrointestinal symptoms. In: Watson M, Campbell R, Vallath N, Ward S, Wells J (eds) Oxford handbook of palliative care, 3rd edn. Oxford University Press, Oxford, pp 317–363
De Conno F, Martini C, Sbanotto A, Ripamonti C, Ventafridda V (2010) Mouth care. In: Hanks G, Cherny NI, Christakis NA, Fallon M, Kaasa S, Portenoy RK (eds) Oxford textbook of palliative medicine, 4th edn. Oxford University Press, Oxford, pp 996–1014
Epstein JB, Güneri P, Barasch A (2014) Appropriate and necessary oral care for people with cancer: guidance to obtain the right oral and dental care at the right time. Support Care Cancer 22:1981–1988
Ohno T, Morita T, Tamura F, Hirano H, Watanabe Y, Kikutani T (2016) The need and availability of dental services for terminally ill cancer patients: a nationwide survey in Japan. Support Care Cancer 24:19–22
Walls AW, Murray ID (1993) Dental care of patients in a hospice. Palliat Med 7:313–321
Fowell A, Finlay I, Johnstone R, Minto L (2002) An integrated care pathway for the last two days of life: Wales-wide benchmarking in palliative care. Int J Palliat Nurs 8:566–573
McCann RM, Hall WJ, Groth-Juncker A (1994) Comfort care for terminally ill patients: the appropriate use of nutrition and hydration. JAMA 272:1263–1266
Coulter S, Gray R, Watson M (2006) The need for dental involvement in palliative care. Eur J Palliat Care 13:94–97
Sweeney MP, Bagg J, Baxter WP, Aitchison TC (1997) Clinical trial of a mucin-containing oral spray for treatment of xerostomia in hospice patients. Palliat Med 11:225–232
Davies AN, Daniels C, Pugh R, Sharma K (1998) A comparison of artificial saliva and pilocarpine in the management of xerostomia in patients with advanced cancer. Palliat Med 12:105–111
Davies AN (2000) A comparison of artificial saliva and chewing gum in the management of xerostomia in patients with advanced cancer. Palliat Med 14:197–203
Monsen RE, Herlofson BB, Gay C, Fjeld KG, Hove LH, Malterud KE et al (2021) A mouth rinse based on a tea solution of Salvia officinalis for oral discomfort in palliative cancer care: a randomized controlled trial. Support Care Cancer 29:4997–5007
Davies AN, Broadley K, Beighton D (2001) Xerostomia in patients with advanced cancer. J Pain Symptom Manage 22:820–825
Hanchanale S, Adkinson L, Daniel S, Fleming M, Oxberry SG (2015) Systematic literature review: xerostomia in advanced cancer patients. Support Care Cancer 23:881–888
Rydholm M, Strang P (1999) Acupuncture for patients in hospital-based home care suffering from xerostomia. J Palliat Care 15:20–23
Wu X, Chung VC, Hui EP, Ziea ET, Ng BF, Ho RS et al (2015) Effectiveness of acupuncture and related therapies for palliative care of cancer: overview of systematic reviews. Sci Rep 5:16776
Nikles J, Mitchell GK, Hardy J, Agar M, Senior H, Carmont SA et al (2015) Testing pilocarpine drops for dry mouth in advanced cancer using n-of-1 trials: a feasibility study. Palliat Med 29:967–974
Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R (2011) Inteventions for the management of dry mouth: topical therapies. Cochrane Database of Systematic Reviews 12. CD008934
Furness S, Bryan G, McMillan R, Birchenough S, Worthington HV (2013) Interventions for the management of dry mouth: non-pharmacological interventions. Cochrane Database of Systematic Reviews 9 CD009603.
Bovio G, Montagna G, Bariani C, Baiardi P (2009) Upper gastrointestinal symptoms in patients with advanced cancer: relationship to nutritional and performance status. Support Care Cancer 17:1317–1324
Brisbois TD, de Kock IH, Watanabe SM, Baracos VE, Wismer WV (2011) Characterization of chemosensory alterations in advanced cancer reveals specific chemosensory phenotypes impacting dietary intake and quality of life. J Pain Symptom Manage 41:673–683
Mortazavi H, Shafiei S, Sadr S, Safiaghdam H (2018) Drug-related dysgeusia: a systematic review. Oral health Prev Dent 16:499–507
Epstein JB, Barasch A (2010) Taste disorders in cancer patients: pathogenesis, and approach to assessment and management. Oral Oncol 46:77–81
Epstein JB, de Andrade E, Silva SM, Epstein GL, Leal JH, Barasch A, Smutzer G (2019) Taste disorders following cancer treatment: report of a case series. Support Care Cancer 27:4587–4595
Wismer WV (2008) Assessing alterations in taste and their impact on cancer care. Curr Opin Support Palliat Care 2:282–287
Boltong A, Keast RS, Aranda SK (2011) A matter of taste: making the distinction between taste and flavor is essential for improving management of dysgeusia. Support Care Cancer 19:441–442
Kumbargere Nagraj S, George RP, Shetty N, Levenson D, Ferraiolo DM, Shrestha A, Interventions for managing taste disturbances. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No.: CD010470.
Brisbois TD, de Kock IH, Watanabe SM, Mirhosseini M, Lamoureux DC, Chasen M et al (2011) Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double-blind, placebo-controlled pilot trial. Ann Oncol 22:2086–2093
Thorne T, Olson K, Wismer W (2015) A state-of-the-art review of the management and treatment of taste and smell alterations in adult oncology patients. Support Care Cancer 23:2843–2851
Oneschuk D, Hanson J, Bruera E (2000) A survey of mouth pain and dryness in patients with advanced cancer. Support Care Cancer 8:372–376
Farquhar-Smith P, Epstein J (2010) Orofacial pain. In: Davies AN, Epstein JB (eds) Oral complications of cancer and its management. Oxford University Press, Oxford, pp 241–251
Epstein JB, Elad S, Eliav E, Jurevic R, Benoliel R (2007) Orofacial pain in cancer: part II - clinical perspectives and management. J Dent Res 86:506–518
Quirynen M, Zhao H, van Steenberghe D (2002) Review of the treatment strategies for oral malodour. Clin Oral Investig 6:1–10
Kumbargere Nagraj S, Eachempati P, Uma E, Singh VP, Ismail NM, Varghese E. Interventions for managing halitosis. Cochrane Database of Systematic Reviews 2019, Issue 12. Art.No.: CD012213.
Samaranayake LP (1990) Host factors and oral candidosis. In: Samaranayake LP, MacFarlane TW (eds) Oral candidosis. Wright, London, pp 66–103
Lalla RV, Latortue MC, Hong CH, Ariyawardana A, D’Amato-Palumbo S, Fischer DJ et al (2010) A systematic review of oral fungal infections in patients receiving cancer therapy. Support Care Cancer 18:985–992
Davies A, Brailsford S, Broadley K, Beighton D (2002) Resistance amongst yeasts isolated from the oral cavities of patients with advanced cancer. Palliat Med 16:527–531
Bagg J, Sweeney MP, Lewis MA, Jackson MS, Coleman D, Al MA et al (2003) High prevalence of non-albicans yeasts and detection of anti-fungal resistance in the oral flora of patients with advanced cancer. Palliat Med 17:477–481
Astvad K, Johansen HK, Høiby N, Steptoe P, Ishøy T (2015) Oropharyngeal candidiasis in palliative care patients in Denmark. J Palliat Med 18:940–944
Lagman R, Davis M, LeGrand S, Walsh D, Parala A, Gamier P et al (2017) Single-dose fluconazole therapy for oral thrush in hospice and palliative medicine patients. Am J Hosp Palliat Care 34:645–649
Ellepola AN, Samaranayake LP (2001) Adjunctive use of chlorhexidine in oral candidoses: a review. Oral Dis 7:11–17
Bagg J, Jackson MS, Sweeney MP, Ramage G, Davies AN (2006) Susceptibility to Melaleuca alternifolia (tea tree) oil of yeasts isolated from the mouths of patients with advanced cancer. Oral Oncol 42:487–492
Funding
Open Access funding provided by the IReL Consortium
Author information
Authors and Affiliations
Contributions
Prof Davies led the project, screened the papers, extracted data from the papers and wrote the initial/subsequent drafts of the guidance. Dr Jones performed the literature searches, screened the papers, extracted data from the papers and contributed to the drafts of the guidance. All the other authors screened the papers and contributed to the drafts of the guidance. All authors approved the final version of the guidance.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1 MEDLINE search strategy
-
1.
Oral care—keyword
or (search terms 2–56)
-
2.
Oral medicine—MESH
-
3.
Dental care—MESH
-
4.
Oral health—MESH
-
5.
Oral assessment—keyword
-
6.
Oral hygiene—MESH
-
7.
Toothbrushing—MESH
-
8.
Interdental cleaning—keyword
-
9.
Mouthwashes—MESH
-
10.
Mouth—MESH
-
11.
Mouth diseases—MESH
-
12.
Lip diseases—MESH
-
13.
Cheilitis—MESH
-
14.
Tongue diseases—MESH
-
15.
Mouth mucosa—MESH
-
16.
Oral mucosa—keyword
-
17.
Stomatitis—MESH
-
18.
Oral ulcer—MESH
-
19.
Tooth—MESH
-
20.
Tooth diseases—MESH
-
21.
Toothache—MESH
-
22.
Dentin sensitivity—MESH
-
23.
Tooth erosion—MESH
-
24.
Dental plaque—MESH
-
25.
Dentures—MESH
-
26.
Dental prosthesis—MESH
-
27.
Denture retention—MESH
-
28.
Stomatitis, denture—MESH
-
29.
Dental devices, home care—MESH
-
30.
Jaw diseases—MESH
-
31.
Mastication—MESH
-
32.
Trismus—MESH
-
33.
Salivary gland diseases—MESH
-
34.
Xerostomia—MESH
-
35.
Dry mouth—keyword
-
36.
Taste disorders—MESH
-
37.
Taste disturbance—keyword
-
38.
Ageusia—MESH
-
39.
Dysgeusia—MESH
-
40.
Hypogeusia—keyword
-
41.
Facial pain—MESH
-
42.
Jaw pain—keyword
-
43.
Oral pain—keyword
-
44.
Mouth pain—keyword
-
45.
Glossalgia—MESH
-
46.
Burning mouth syndrome—MESH
-
47.
Oral infections—keyword
-
48.
Oral bacterial infections—keyword
-
49.
Dental caries—MESH
-
50.
Periodontal diseases—MESH
-
51.
Gingivitis—MESH
-
52.
Oral fungal infections—keyword
-
53.
Candidiasis, oral—MESH
-
54.
Oral candidosis—keyword
-
55.
Oral viral infections—keyword
-
56.
Halitosis—MESH
and (search terms 57–58)
-
57.
Neoplasm—MESH
or
-
58.
Cancer—keyword
Appendix 2 MASCC criteria for grading recommendations [6]
Levels of evidence.
I | Evidence obtained from meta-analysis of multiple, well-designed, controlled studies; randomised trials with low false-positive and false-negative errors (high power) |
II | Evidence obtained from at least one well-designed experimental study; randomised trials with high false-positive and/or false-negative errors (low power) |
III | Evidence obtained from well-designed, quasi-experimental studies, such as nonrandomized, controlled single-group, pretest–posttest comparison, cohort, time or matched case–control series |
IV | Evidence obtained from well-designed, non-experimental studies, such as comparative and correlational descriptive and case studies |
V | Evidence obtained from case reports and clinical examples |
Categories of guidelines.
Recommendation | Reserved for guidelines that are based on Level I or Level II evidence |
Suggestion | Used for guidelines that are based on Level III, Level IV and Level V evidence; this implies panel consensus on the interpretation of this evidence |
No guideline possible | Used when there is insufficient evidence on which to base a guideline; this implies (1) that there is little or no evidence regarding the practice in question, or (2) that the panel lacks consensus on the interpretation of existing evidence |
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jones, J.A., Chavarri-Guerra, Y., Corrêa, L.B.C. et al. MASCC/ISOO expert opinion on the management of oral problems in patients with advanced cancer. Support Care Cancer 30, 8761–8773 (2022). https://doi.org/10.1007/s00520-022-07211-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07211-2