Abstract
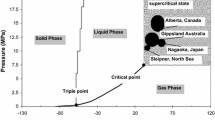
The determination of effective carbon dioxide (CO2) permeability in reservoir rock and its variation is of great interest in the process of CO2 sequestration in deep saline aquifers, as CO2 sequestration-induced permeability alternations appear to create major problems during the CO2 injection process. The main objective of this study is to investigate the effect of salinity on the effective CO2 permeability of reservoir rock under different injection pressures. A series of high-pressure tri-axial experiments was, therefore, performed to investigate the effect of salinity on effective CO2 permeability in Hawkesbury sandstone under various brine concentrations. The selected brine concentrations were 0, 10, 20, and 30 % sodium chloride (NaCl) by weight and the experiments were conducted for a range of CO2 injection pressures (2, 4, 6, 8, 10, and 12 MPa) at a constant confinement of 20 MPa and a temperature of 35 °C, respectively. According to the results, the degree of salinity of the aquifer’s pore fluid plays a vital role in the effective CO2 permeability variation which occurs with CO2 injection, and the effective permeability decreases with increasing salinity in the range of 0–30 % of NaCl. Interestingly, in dry reservoir rock samples, the phase transition of the injection of CO2 from gas to super-critical condition caused a sudden reduction of CO2 permeability, related to the slip flow effect which occurs in gas CO2. Transfer into vapor or super-critical CO2 causes this slip flow to be largely reduced, reducing the reservoir permeability for CO2 movement in dry reservoir rock samples. However, this behavior was not observed for water- and brine-saturated samples, and an increasing trend of effective CO2 permeability was observed with increasing injection pressure. A detailed chemical analysis was then conducted to understand the physical phenomenon causing the salinity effect on effective CO2 permeability using scanning electron microscopy analyses. Such analyses explain the reason for the observed permeability variations by giving detailed images of the rock sample’s microstructure. There were clear depositions of NaCl crystals in the rock’s pore space, and the amount increased with increasing brine concentration.



















Similar content being viewed by others
References
Abbas A, Carcasses M, Ollivier JP (1999) Gas permeability of concrete in relation to its degree of saturation. Mater Struct 32:3–8
Aktan T, Farouk ASM (1975) Effect of cyclic and in situ heating on the absolute permeabilities, elastic constants and electrical resistivities of rocks. Presented at the 50th Annual Fall Meeting of the SPE of AIME, Dallas, TX SPE, 5633:1–10
Arsyad A, Mitani Y, Babadagli T (2013) Comparative assessment of potential ground uplift induced by injection of CO2 into Ainoura, and Berea sandstone formations. Procedia Earth Planet Sci 6:278–286
Bachu S, Bennion B (2008) Effects of in situ conditions on relative permeability characteristics of CO2-brine systems. Environ Geol 54(8):1707–1722
Baudracco J, Tardy Y (1988) Dispersion and flocculation of clays in unconsolidated sandstone reservoirs subjected to percolation with NaCl and CaCl2 solutions at different temperatures. Appl Clay Sci 3(4):347–360
Bennion B, Bachu S. (2005). Relative permeability characteristics for CO2 displacing water in a variety of potential sequestration zones in the Western Canada Sedimentary Basin. Paper SPE 95547, p 15, presented at the 2005 SPE Technical Conference and Exhibition, Dallas, TX, 9–12 October 2005 PAGES?
Bennion B, Bachu S (2006c) Dependence on temperature, pressure and salinity of the IFT and relative permeability displacement characteristics of CO2 injected in deep saline aquifers. Paper SPE 102138, p 10, presented at the 2006 SPE technical conference and exhibition, San Antonio, TX, 24–27 September : 120–133
Bennion DB, Bachu S (2010) Drainage and imbibition CO2/brine relative permeability curves at reservoir conditions for carbonate formations, in SPE Annual technical conference and exhibition, SPE 134028, pp 1–18, Society of Petroleum Engineers, Florence, Italy
Berg S, Oedai S, Ott H (2013) Displacement and mass transfer between saturated and unsaturated CO2-brine systems in sandstone. Int J Greenh Gas Control 12:478–492
Biot MA (1941) General theory of three-dimensional consolidation. J Appl Phys 12(2):155–164
Burton M, Kumar N, Bryant SL (2009) CO2 injectivity into brine aquifers: why relative permeability matters as much as absolute permeability. Energy Procedia 1(1):3091–3098
Chalbaud C, Robin M, Egermann P (2006) Interfacial tension of CO2/brine systems at reservoirs conditions. In: Gale J, Rokke N, Zweigel P, Swenson H (eds) Proceedings of the 8th International Conference on Greenhouse Gas Control Technology, Elsevier, Amsterdam CD ROM
Chen Y, Zhou C, Sheng Y (2007) Formulation of strain-dependent hydraulic conductivity for a fractured rock mass. Int J Rock Mech Min Sci 44(7):981–996
Cnudde V, Cwirzen A, Masschaele B, Jacobs PJS (2009) Porosity and microstructural characterization of building stones and concretes. Eng Geol 103(3–4):76–83
Dahab AE, El Omar, Gassier MM, Kariem HA (1992) Formation damage effects due to salinity, temperature and pressure in sandstone reservoirs as indicated by relative permeability measurements. J Petrol Sci Eng 6(4):403–412
Davies JP, Davies DK (1999) Stress-dependent permeability: characterization and modeling, Soc Pet Eng (SPE), Houston
Dong JJ, Hsu JY, Wu WJ, Shimamoto T, Hung JH, Yeh EC et al (2010) Stress-dependence of the permeability and porosity of sandstone and shale from TCDP Hole-A. Int J Rock Mech Min Sci 47(7):1141–1157
Faulkner D, Rutter E (2000) Comparisons of water and argon permeability in natural clay‐bearing fault gouge under high pressure at 20 °C. J Geophys Res Solid Earth (1978–2012) 105(B7):16415–16426
Fenghou A, Wakeham WA, Vesovic V (1998) The viscosity of carbon dioxide. J Phys-Chem Ref Data 27:31–44
Hangx S, Van der Linden A, Marcellis F, Bauer A (2013) The effect of CO2 on the mechanical properties of the Captain Sandstone: geological storage of CO2 at the Goldeneye field (UK). Int J Greenh Gas Control 201:5–17
Jasinge D, Ranjith PG, Choi SK (2011) Effects of effective stress changes on permeability of Latrobe Valley brown coal. Fuel 90(3):1292e 300
Jing XD, Archer JS, Daltaban TS (1992) Laboratory study of the electrical and hydraulic properties of rocks under simulated reservoir conditions. Mar Pet Geol 9(2):115–127
Juanes R, Spiteri EJ, Orr FM, Blunt MJ (2006) Impact of relative permeability hysteresis on geological CO2 storage. Water Resour Res 42(12):32–41
Julio GE (2001) Density of aqueous solutions of CO2. Lawrence Berkeley National Laboratory. http://escholarship.org/uc/item/6dn022hb
Keaney GM, Meredith P, Murrell S, Barker J (2004) Determination of the effective stress laws for permeability and specific storage in low porosity sandstone. Gulf Rocks 2004, the 6th North America rock mechanics Symposium (NARMS), 5–9 June, Texas, Houston, pp 24–36
Khilar KC (1981) Water sensitivity of sandstone. Ph.D thesis, University of Michigan
Klinkenberg L (1941) The permeability of porous media to liquids and gases. Drilling and production practice. American Petroleum Institute, New York, pp 21–34
Kuhn M, Vernoux JF, Kellner T, Isenbeck-Schroter M, Schulz HD (1998) Onsite experimental simulation of brine injection into a clastic reservoir as applied to geothermal exploitation in Germany. Appl Geochem 13:477–490
Leverett M (1940) Capillary behavior in porous solids. Trans. Am. Inst. Pet Transp 12:123–134
Loosveldt H, Lafhaj Z, Skoczylas F (2002) Experimental study of gas and liquid permeability of a mortar. Cem Concr Res 32:1357–1363
Miller MA, Ramey HJ (1986) Effect of temperature on oil/water relative permeability of unconsolidated sands. J Pet Eng 34:1945–1955
Mohan KK, Reed MG, Fogler HS (1999) Formation damage in smectic sandstones by high ionic strength brines. Colloids and surfaces A: physical and Chemical Engineering. Aspects 154:249–257
Molina E, Cultrone G, Sebastian E, Alonso FJ, Carrizo L, Gisbert J, Buj O (2011) The pore system of sedimentary rocks as a key factor in the durability of building materials. Eng Geol 118(3–4):110–121
Monlouis-Bonnaire JP, Verdier J, Perrin B (2004) Prediction of the relative permeability to gas flow of cement-based materials. Cement and Concrete Research 34:737–744
Nasvi MCM, Ranjith PG, Sanjayan J (2013) The permeability of geopolymer at down-hole stress conditions: application for carbón dioxide sequestration Wells. Appl Energy 102:1391–1398
Neuzil CE (1994) How permeable are clays and shales? Water Resour Res 30(2):145–150
Ochi J, Vernoux JF (1998) Permeability decrease in sandstone reservoirs by fluid injection: hydrodynamic and chemical effects. J Hydrol 208:237–248
Odeh AS (1959) Effect of viscosity ratio on relative permeability. Pet Transp AIME 216:346–353
Omar A (1990) Effect of brine composition and clay content on the permeability damage of sandstone cores. J Pet Sci Eng 4(3):245–256
Pearce JM, Holloway S, Wacker H, Nelis MK, Rochelle C, Bateman K (1996) Natural occurrences as analogues for the geological disposal of carbon dioxide. Energy Convers Manag 37(6–8):1123–1128
Perera MSA, Ranjith PG, Airey D, Choi SK (2011) Sub-critical and super-critical carbon dioxide flow behaviour in naturally fractured black coal: an experimental study. J Fuel. doi:10.1016/j.fuel.2011.05.016
Picandet V, Khelidj A, Bellegou H (2009) Crack effects on gas and water permeability of concretes. Cem Concr Res 39:537–547
Probst P (2008) Numerical simulations of CO2 injection into saline aquifers: estimation of storage capacity and arrival times using multiple realizations of heterogeneous permeability fields. Master thesis, University of Stuttgart, German
Ranjith PG, Perera MSA (2011) A new triaxial apparatus to study the mechanical and fluid flow aspects of carbon dioxide sequestration in geological formations. Fuel 90:2751–2759
Ranjith PG, Perera MSA, Khan E (2013) A study of safe CO2 storage capacity in saline aquifers: a numerical study. Int J Energy Res 37:189–199
Rathnaweera TD, Ranjith PG, Perera MSA (2014) Salinity-dependent strength and stress-strain characteristics of reservoir rocks in deep saline aquifers: an experimental study. Fuel 122:1–11
Rutqvist J, Tsang CF (2002) A study of caprock hydro-mechanical changes associated with CO2-injection into a brine formation. Environ Geol 42:296–305
Samuel CMK, Ronny P, Zuo L, Benson SM (2012) Relative permeability and trapping of CO2 and water in sandstone rocks at reservoir conditions. Water Resour Res 48:w02532. doi:10.1029/2011wr010859
Scheidegger AE (1958) The physics of flow through porous media. Soil Sci 86(6):355–342
Shukla R (2011) Study of reservoir rock and caprock integrity in geo-sequestration of carbon dioxide. Ph. D. thesis. Monash University, Australia
Shukla R, Ranjith PG, Choi SK, Haque A, Yellishetty M, Hong L (2012) Mechanical behaviour of reservoir rock under brine saturation. Rock Mech Rock Eng 46(1):83–89
Siriwardane HJ, Gondle RK, Smith DH (2009) Influence of carbon dioxide on coal permeability determined by pressure transient methods. Int J Coal Geol 77(1):109–118
Sydansk RD (1981) Discussion of the effect of temperature and confining pressure on single-phase flow in consolidated rocks. J Pet Eng 32:1329–1330
Tanikawa W, Shimamoto T (2009) Comparison of Klinkenberg-corrected gas permeability and water permeability in sedimentary rocks. Int J Rock Mech Min Sci 46(2):229–238
Vilarrasa V, Olivella S, Carrera J (2010) Geomechanical stability of the caprock during CO2 sequestration in deep saline aquifers. Energy 4:5306–5313
Wang HF (2000) Theory of linear poroelasticity: with applications to geomechanics and hydrogeology. Princeton University Press, Princeton
Ward CR (1972) Sedimentation in the Narrabeen Group, southern Sydney basin, New South Wales. J Geol Soc Australia 19(3):393–409
Wegener DC, Harpole KJ (2010) Determination of relative permeability and trapped gas saturation for predictions of WAG performance in the South Cowden CO2 flood, in SPE/DOE tenth symposium on improved oil recovery, SPE 35429, Society of Petroleum Engineers, Tulsa, Oklahoma, pp 1–13
White CU, Strazisar BR, Granite EJ, Hoffman JS, Pennline HW (2003) Separation and capture of CO2 from large stationary sources and sequestration in geological formations—coalbeds and deep saline aquifers. J Air Waste Manag Assoc 53(6):645–715. doi:10.1080/10473289.2003.10466206
Yang D, Tontiwachwuthikul P, Gu Y (2005) Interfacial tensions of the crude oil + reservoir brine + CO2 systems at pressures up to 31 MPA and temperatures of 27°C and 58°C. J Chem Eng Data 50:1242–1249
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rathnaweera, T.D., Ranjith, P.G. & Perera, M.S.A. Effect of Salinity on Effective CO2 Permeability in Reservoir Rock Determined by Pressure Transient Methods: an Experimental Study on Hawkesbury Sandstone. Rock Mech Rock Eng 48, 2093–2110 (2015). https://doi.org/10.1007/s00603-014-0671-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00603-014-0671-0