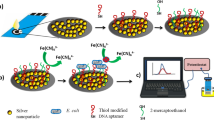
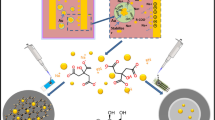
Abstract
In this work, novel silver sulphide quantum dots (Ag2S QD) are electrochemically quantified for the first time. The method is based on the electrochemical reduction of Ag+ to Ag0 at −0.3 V on screen-printed carbon electrodes (SPCEs), followed by anodic stripping voltammetric oxidation that gives a peak of currents at +0.06 V which represents the analytical signal. The optimized methodology allows the quantification of water-stabilized Ag2S QD in the range of approximately 2 × 109–2 × 1012 QD·mL−1 with a good reproducibility (RSD: 5%). Moreover, as proof-of-concept of relevant biosensing application, Ag2S QD are evaluated as tags for Escherichia coli (E. coli) bacteria determination. Bacteria tagged with QD are separated by centrifugation from the sample solution and placed on the SPCE surface for quantitative analysis. The effect of two different Ag2S QD surface coating/stabilizing agents on both the voltammetric response and the bacteria sensing is also evaluated. 3-mercaptopropionic acid (3-MPA) is studied as model of short length coating ligand with no affinity for the bacteria, while boronic acid (BA) is evaluated as longer length ligand with chemical affinity for the polysaccharides present in the peptidoglycan layer on the bacteria cells surface. The biosensing system allows to detect bacteria in the range 10−1-103 bacteria·mL−1 with a limit of detection as low as 1 bacteria·mL−1. This methodology is a promising proof-of-concept alternative to traditional laboratory-based tests, with good sensitivity and short time and low cost of analysis.

Novel silver sulphide quantum dots (Ag2S QD) are electrochemically quantified for the first time. Moreover, Ag2S QD are evaluated as tags for Escherichia coli bacteria determination. The effect of two different QD surface coating ligands is also evaluated.





Similar content being viewed by others
References
Editorial (2014) Nanocrystals in their prime. Nat Nanotechnol 9:325. https://doi.org/10.1038/nnano.2014.101
Ekimov AI, Onushchenko AA (1982) Quantum size effect in 3D microscopic semiconductor crystals. JETP Lett 34:345–348
Alivisatos AP (1996) Semiconductor clusters, nanocrystals, and quantum dots. Science 271(80):933–937
Murray CB, Norris DJ, Bawendi MG (1993) Synthesis and characterization of nearly Monodisperse CdE (E = S, Se, Te) semiconductor Nanocrystallites. J Am Chem Soc 115:8706–8715. https://doi.org/10.1021/ja00072a025
Grabolle M, Ziegler J, Merkulov A, Nann T, Resch-Genger U (2008) Stability and fluorescence quantum yield of CdSe-ZnS quantum dots - influence of the thickness of the ZnS shell. Ann N Y Acad Sci 1130:235–241. https://doi.org/10.1196/annals.1430.021
Montoro Bustos AR, Trapiella-Alfonso L, Ruiz Encinar J, Costa-Fernández JM, Pereiro R, Sanz-Medel A (2012) Elemental and molecular detection for quantum dots-based immunoassays: a critical appraisal. Biosens Bioelectron 33:165–171. https://doi.org/10.1016/j.bios.2011.12.046
Trapiella-Alfonso L, Montoro Bustos AR, Ruiz Encinar J, Costa-Fernández JM, Pereiro R, Sanz-Medel A (2011) New integrated elemental and molecular strategies as a diagnostic tool for the quality of water soluble quantum dots and their bioconjugates. Nanoscale 3:954–957. https://doi.org/10.1039/c0nr00822b
Montoro Bustos AR, Ruiz Encinar J, Fernández-Argüelles MT, Costa-Fernández JM, Sanz-Medel A (2009) Elemental mass spectrometry: a powerful tool for an accurate characterisation at elemental level of quantum dots. Chem Commun 3107–3109. https://doi.org/10.1039/b901493d
Amelia M, Impellizzeri S, Monaco S, Yildiz I, Silvi S, Raymo FM, Credi A (2011) Structural and size effects on the spectroscopic and redox properties of CdSe nanocrystals in solution: the role of defect states. ChemPhysChem 12:2280–2288. https://doi.org/10.1002/cphc.201100300
Haram SK, Quinn BM, Bard AJ (2001) Electrochemistry of CdS nanoparticles: a correlation between optical and electrochemical band gaps. J Am Chem Soc 123:8860–8861. https://doi.org/10.1021/ja0158206
Bard AJ, Ding Z, Myung N (2005) Electrochemistry and electrogenerated chemiluminescence of semiconductor nanocrystals in solutions and in films. Struct Bond 118:1–57. https://doi.org/10.1007/b137239
Wang J, Liu G, Polsky R, Merkoçi A (2002) Electrochemical stripping detection of DNA hybridization based on cadmium sulfide nanoparticle tags. Electrochem Commun 4:722–726. https://doi.org/10.1016/S1388-2481(02)00434-4
de la Escosura-Muñiz A, Ambrosi A, Merkoçi A (2008) Electrochemical analysis with nanoparticle-based biosystems. TrAC - Trends Anal Chem 27:568–584. https://doi.org/10.1016/j.trac.2008.05.008
Zhao P, Xu Q, Tao J, Jin Z, Pan Y, Yu C, Yu Z (2018) Near infrared quantum dots in biomedical applications: current status and future perspective. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 10:1–16. https://doi.org/10.1002/wnan.1483
Gu YP, Cui R, Zhang ZL, Xie ZX, Pang DW (2012) Ultrasmall near-infrared Ag 2Se quantum dots with tunable fluorescence for in vivo imaging. J Am Chem Soc 134:79–82. https://doi.org/10.1021/ja2089553
Llano-Suárez P, Bouzas-Ramos D, Costa-Fernández JM, Soldado A, Fernández-Argüelles MT (2019) Near-infrared fluorescent nanoprobes for highly sensitive cyanide quantification in natural waters. Talanta 192:463–470. https://doi.org/10.1016/j.talanta.2018.09.073
Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai WR, Rodriguez W, Bassett IV (2014) Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 14:239–249. https://doi.org/10.1016/S1473-3099(13)70250-0
WHO (2017) Global action plan on antimicrobial resistance. Who 1–28
Hermanson GT (2013) Bioconjugate techniques, Third Edit. Elsevier Inc., London
Espinoza-Castañeda M, de la Escosura-Muñiz A, González-Ortiz G, Martín-Orúe SM, Pérez JF, Merkoçi A (2013) Casein modified gold nanoparticles for future theranostic applications. Biosens Bioelectron 40:271–276. https://doi.org/10.1016/j.bios.2012.07.042
Tamerat N, Muktar Y (2016) Application of molecular diagnostic techniques for the detection of E. coli O157:H7: a review. J Vet Sci Technol 7. https://doi.org/10.4172/2157-7579.1000362
Sepunaru L, Tschulik K, Batchelor-McAuley C, Gavish R, Compton RG (2015) Electrochemical detection of single E. coli bacteria labeled with silver nanoparticles. Biomater Sci 3:816–820. https://doi.org/10.1039/c5bm00114e
Feng ZV, Gunsolus IL, Qiu TA, Hurley KR, Nyberg LH, Frew H, Johnson KP, Vartanian AM, Jacob LM, Lohse SE, Torelli MD, Hamers RJ, Murphy CJ, Haynes CL (2015) Impacts of gold nanoparticle charge and ligand type on surface binding and toxicity to gram-negative and gram-positive bacteria. Chem Sci 6:5186–5196. https://doi.org/10.1039/c5sc00792e
Wu X, Lai T, Jiang J, Ma Y, Tao G, Liu F, Li N (2019) An on-site bacterial detection strategy based on broad-spectrum antibacterial ε-polylysine functionalized magnetic nanoparticles combined with a portable fluorometer. Microchim Acta 186:526. https://doi.org/10.1007/s00604-019-3632-1
Russo L, Leva Bueno J, Bergua JF, Costantini M, Giannetto M, Puntes V, de la Escosura-Muñiz A, Merkoçi A (2018) Low-cost strategy for the development of a rapid electrochemical assay for bacteria detection based on AuAg nanoshells. ACS Omega 3:18849–18856. https://doi.org/10.1021/acsomega.8b02458
Hassan A-RHA-A, de la Escosura-Muñiz A, Merkoçi A (2015) Highly sensitive and rapid determination of Escherichia coli O157:H7 in minced beef and water using electrocatalytic gold nanoparticle tags. Biosens Bioelectron 67:511–515. https://doi.org/10.1016/j.bios.2014.09.019
Resendez A, Halim MA, Singh J, Webb DL, Singaram B (2017) Boronic acid recognition of non-interacting carbohydrates for biomedical applications: increasing fluorescence signals of minimally interacting aldoses and sucralose. Org Biomol Chem 15:9727–9733. https://doi.org/10.1039/c7ob01893b
Li J, Wu H, Santana I, Fahlgren M, Giraldo JP (2018) Standoff optical glucose sensing in photosynthetic organisms by a quantum dot fluorescent probe. ACS Appl Mater Interfaces 10:28279–28289. https://doi.org/10.1021/acsami.8b07179
Whyte GF, Vilar R, Woscholski R (2013) Molecular recognition with boronic acids-applications in chemical biology. J Chem Biol 6:161–174. https://doi.org/10.1007/s12154-013-0099-0
Chen J, Andler SM, Goddard JM, Nugen SR, Rotello VM (2017) Integrating recognition elements with nanomaterials for bacteria sensing. Chem Soc Rev 46:1272–1283. https://doi.org/10.1039/c6cs00313c
Miranda OR, Li X, Garcia-Gonzalez L, Zhu ZJ, Yan B, Bunz UH, Rotello VM (2011) Colorimetric bacteria sensing using a supramolecular enzyme-nanoparticle biosensor. J Am Chem Soc 133:9650–9653. https://doi.org/10.1021/ja2021729
Chen J, Jiang Z, Ackerman JD, Yazdani M, Hou S, Nugen SR, Rotello VM (2015) Electrochemical nanoparticle-enzyme sensors for screening bacterial contamination in drinking water. Analyst 140:4991–4996. https://doi.org/10.1039/c5an00637f
Sun J, Huang J, Li Y, Lv J, Ding X (2019) A simple and rapid colorimetric bacteria detection method based on bacterial inhibition of glucose oxidase-catalyzed reaction. Talanta 197:304–309. https://doi.org/10.1016/j.talanta.2019.01.039
Singh AK, Senapati D, Wang S, Griffin J, Neely A, Candice P, Naylor KM, Varisli B, Kalluri JR, Ray PC (2009) Gold Nanorod based selective identification of Escherichia coli Bacteria using two-photon Rayleigh scattering spectroscopy. ACS Nano 3:1906–1912
Wang C, Irudayaraj J (2008) Gold nanorod probes for the detection of multiple pathogens. Small 4:2204–2208. https://doi.org/10.1002/smll.200800309
Wang S, Singh AK, Senapati D, Neely A, Yu H, Ray PC (2010) Rapid colorimetric identification and targeted photothermal lysis of Salmonella bacteria by using bioconjugated oval-shaped gold nanoparticles. Chem - A Eur J 16:5600–5606. https://doi.org/10.1002/chem.201000176
Joung CK, Kim HN, Im HC, Kim HY, Oh MH, Kim YR (2012) Ultra-sensitive detection of pathogenic microorganism using surface-engineered impedimetric immunosensor. Sensors Actuators B Chem 161:824–831. https://doi.org/10.1016/j.snb.2011.11.041
Zuo P, Li X, Dominguez DC, Ye BC (2013) A PDMS/paper/glass hybrid microfluidic biochip integrated with aptamer-functionalized graphene oxide nano-biosensors for one-step multiplexed pathogen detection. Lab Chip 13:3921–3928. https://doi.org/10.1039/c3lc50654a
Duan YF, Ning Y, Song Y, Deng L (2014) Fluorescent aptasensor for the determination of Salmonella typhimurium based on a graphene oxide platform. Microchim Acta 181:647–653. https://doi.org/10.1007/s00604-014-1170-4
Acknowledgements
This work has been supported by the FC-GRUPIN-ID/2018/000166 project from the Asturias Regional Government and the CTQ2017–86994-Rand CTQ2016–79412-P projects from the MINECO (Spain). O. Amor-Gutiérrez thanks the University of Oviedo for the award of a grant “Ayudas para la realización de tesis doctorales” (PAPI-18-PF-13). A. Iglesias-Mayor thanks the MECD (Spain) for the award of a FPU Grant (FPU2014/04686). P. Llano-Suárez thanks the University of Oviedo for the award of a grant "Ayudas para la realización de tesis doctorales" (PAPI-18-PF-08). A. de la Escosura-Muñiz acknowledges the MICINN (Spain) for the “Ramón y Cajal” Research Fellow (RyC-2016-20299) and the University of Oviedo for the “Ayudas Proyectos Emergentes 2019” project (PAPI-19-EMERG-17). F. Parra laboratory was funded by the Municipality of Ribera de Arriba/La Ribera (Asturias, Spain). Authors would like to acknowledge the technical support provided by Servicios Científico-Técnicos de la Universidad de Oviedo (Alaa Adawy at the laboratory of HR-TEM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In memoriam to Prof. Agustín Costa-García.
This article is part of the Topical Collection on IX NyNA 2019. International Congress on Analytical Nanoscience and Nanotechnology
This work was presented at the IX NyNA 2019, International Congress on Analytical Nanoscience and Nanotechnology at Zaragoza (Spain) from 2–4 July, 2019. Chairman: Dr. Juan R. Castillo.
Electronic supplementary material
ESM 1
(DOCX 2330 kb)
Rights and permissions
About this article
Cite this article
Amor-Gutiérrez, O., Iglesias-Mayor, A., Llano-Suárez, P. et al. Electrochemical quantification of Ag2S quantum dots: evaluation of different surface coating ligands for bacteria determination. Microchim Acta 187, 169 (2020). https://doi.org/10.1007/s00604-020-4140-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4140-z