Abstract
Background
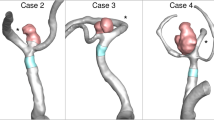
Surgical intervention for unruptured intracranial aneurysms (IAs) carries inherent health risks. The analysis of “patient-specific” IA geometric and computational fluid dynamics (CFD) simulated wall shear stress (WSS) data has been investigated to differentiate IAs at high and low risk of rupture to help clinical decision making. Yet, outcomes vary among studies, suggesting that novel analysis could improve rupture characterization. The authors describe a CFD analytic method to assess spatiotemporal characteristics of swirling flow vortices within IAs to improve characterization.
Methods
CFD simulations were performed for 47 subjects harboring one medium-sized (4–10 mm) middle cerebral artery (MCA) aneurysm with available 3D digital subtraction angiography data. Alongside conventional indices, quantified IA flow vortex spatiotemporal characteristics were applied during statistical characterization. Statistical supervised machine learning using a support vector machine (SVM) method was run with cross-validation (100 iterations) to assess flow vortex-based metrics’ strength toward rupture characterization.
Results
Relying solely on vortex indices for statistical characterization underperformed compared with established geometric characteristics (total accuracy of 0.77 vs 0.80) yet showed improvements over wall shear stress models (0.74). However, the application of vortex spatiotemporal characteristics into the combined geometric and wall shear stress parameters augmented model strength for assessing the rupture status of middle cerebral artery aneurysms (0.85).
Conclusions
This preliminary study suggests that the spatiotemporal characteristics of flow vortices within MCA aneurysms are of value to improve the differentiation of ruptured aneurysms from unruptured ones.




Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CFD:
-
Computational fluid dynamics
- IA:
-
Intracranial aneurysms
- MCA:
-
Middle cerebral artery
- NVS:
-
Normalized vortex surface
- OSI:
-
Oscillatory shear index
- ROC:
-
Receiver operating characteristic
- STA-WSS:
-
Spatiotemporally averaged wall shear stress
- STD-DVO:
-
Standard deviation of degree of volume overlap
- STL:
-
Stereolithography
- SAH:
-
Subarachnoid hemorrhage
- SVM:
-
Support vector machine
- TA-DVO:
-
Temporally averaged degree of volume overlap
- LSA-std:
-
Temporal low shear area standard deviation
- TA-WSSMax:
-
Temporally averaged maximum wall shear stress
- TA-WSSMin:
-
Temporally averaged minimum wall shear stress
- TA-LSA:
-
Temporally averaged low wall shear area
- VtV:
-
Vortex volume to IA volume
- WSS:
-
Wall shear stress
References
Ahn J-M, Oh J-S, Yoon S-M, Shim J-H, Oh H-J, Bae H-G (2017) Procedure-related complications during endovascular treatment of intracranial saccular aneurysms. J Cerebrovasc Endovasc Neurosurg 19:162–170
Alimohammadi M, Sherwood JM, Karimpour M, Agu O, Balabani S, Díaz-Zuccarini V (2015) Aortic dissection simulation models for clinical support: fluid-structure interaction vs. rigid wall models. Biomed Eng Online 14:34. https://doi.org/10.1186/s12938-015-0032-6
Balaguru UM, Sundaresan L, Manivannan J, Majunathan R, Mani K, Swaminathan A, Venkatesan S, Kasiviswanathan D, Chatterjee S (2016) Disturbed flow mediated modulation of shear forces on endothelial plane: a proposed model for studying endothelium around atherosclerotic plaques. Sci Rep 6:27304. https://doi.org/10.1038/srep27304https://www.nature.com/articles/srep27304#supplementary-information
Bijlenga P, Gondar R, Schilling S, Morel S, Hirsch S, Cuony J, Corniola M-V, Perren F, Rüfenacht D, Schaller K (2017) PHASES score for the management of intracranial aneurysm. Stroke 48:2105–2112. https://doi.org/10.1161/STROKEAHA.117.017391
Chalouhi N, Zanaty M, Whiting A, Yang S, Tjoumakaris S, Hasan D, Starke RM, Hann S, Hammer C, Kung D (2015) Safety and efficacy of the pipeline embolization device in 100 small intracranial aneurysms. J Neurosurg 122:1498–1502
Chiu J-J, Chien S (2011) Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91:327–387. https://doi.org/10.1152/physrev.00047.2009
Detmer FJ, Chung BJ, Mut F, Pritz M, Slawski M, Hamzei-Sichani F, Kallmes D, Putman C, Jimenez C, Cebral JR (2018) Development of a statistical model for discrimination of rupture status in posterior communicating artery aneurysms. Acta Neurochir 160:1643–1652
Dolan JM, Kolega J, Meng H (2013) High wall shear stress and spatial gradients in vascular pathology: a review. Ann Biomed Eng 41:1411–1427
Duan Z, Li Y, Guan S, Ma C, Han Y, Ren X, Wei L, Li W, Lou J, Yang Z (2018) Morphological parameters and anatomical locations associated with rupture status of small intracranial aneurysms. Sci Rep 8:6440
Gwilliam MN, Hoggard N, Capener D, Singh P, Marzo A, Verma PK, Wilkinson ID (2009) MR derived volumetric flow rate waveforms at locations within the common carotid, internal carotid, and basilar arteries. J Cereb Blood Flow Metab 29:1975–1982. https://doi.org/10.1038/jcbfm.2009.176
He X, Ku DN (1996) Pulsatile flow in the human left coronary artery bifurcation: average conditions. J Biomech Eng 118:74–82. https://doi.org/10.1115/1.2795948
Hoi Y, Wasserman BA, Xie YJ, Najjar SS, Ferruci L, Lakatta EG, Gerstenblith G, Steinman DA (2010) Characterization of volumetric flow rate waveforms at the carotid bifurcations of older adults. Physiol Meas 31:291–302. https://doi.org/10.1088/0967-3334/31/3/002
Jain K, Jiang J, Strother C, Mardal K-A (2016) Transitional hemodynamics in intracranial aneurysms — comparative velocity investigations with high resolution lattice Boltzmann simulations, normal resolution ANSYS simulations, and MR imaging. Med Phys 43:6186–6198. https://doi.org/10.1118/1.4964793
James G, Witten D, Hastie T, Tibshirani R (2013) An introduction to statistical learning: with applications. Springer, New York
Jeong J, Hussain F (1995) On the identification of a vortex. J Fluid Mech 285:69–94
Jiang J, Strother CM (2013) Interactive decomposition and mapping of saccular cerebral aneurysms using harmonic functions: its first application with “patient-specific” computational fluid dynamics (CFD) simulations. IEEE Trans Med Imaging 32:153–164. https://doi.org/10.1109/TMI.2012.2216542
Jiang P, Liu Q, Wu J, Chen X, Li M, Li Z, Yang S, Guo R, Gao B, Cao Y, Wang S (2018) A novel scoring system for rupture risk stratification of intracranial aneurysms: a hemodynamic and morphological study. Front Neurosci 12. https://doi.org/10.3389/fnins.2018.00596
Jing L, Fan J, Wang Y, Li H, Wang S, Yang X, Zhang Y (2015) Morphologic and hemodynamic analysis in the patients with multiple intracranial aneurysms: ruptured versus unruptured. PLoS One 10:e0132494–e0132494. https://doi.org/10.1371/journal.pone.0132494
Jou L-D, Lee DH, Morsi H, Mawad ME (2008) Wall shear stress on ruptured and Unruptured intracranial aneurysms at the internal carotid artery. Am J Neuroradiol 29:1761–1767. https://doi.org/10.3174/ajnr.A1180
Kocur D, Przybyłko N, Baron J, Rudnik A (2019) Endovascular treatment of small (< 5 mm) unruptured middle cerebral artery aneurysms. Pol J Radiol 84:e198–e204. https://doi.org/10.5114/pjr.2019.84829
Korja M, Lehto H, Juvela S (2014) Lifelong rupture risk of intracranial aneurysms depends on risk factors: a prospective Finnish cohort study. Stroke 45:1958–1963
Kotowski M, Naggara O, Darsaut TE, Nolet S, Gevry G, Kouznetsov E, Raymond J (2013) Safety and occlusion rates of surgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis of the literature from 1990 to 2011. J Neurol Neurosurg Psychiatry 84:42–48
Kulcsár Z, Ugron A, Marosfői M, Berentei Z, Paál G, Szikora I (2011) Hemodynamics of cerebral aneurysm initiation: the role of wall shear stress and spatial wall shear stress gradient. Am J Neuroradiol 32:587–594
Lee G-J, Eom K-S, Lee C, Kim D-W, Kang S-D (2015) Rupture of very small intracranial aneurysms: incidence and clinical characteristics. J Cerebrovasc Endovasc Neurosurg 17:217–222
Leopardi P (2006) A partition of the unit sphere into regions of equal area and small diameter. Electron Trans Numer Anal 25:309–327
Li H, Lin K, Shahmirzadi D (2016) FSI simulations of pulse wave propagation in human abdominal aortic aneurysm: the effects of sac geometry and stiffness. Biomed Eng Comput Biol 7:BECB.S40094. https://doi.org/10.4137/becb.s40094
Liu J, Jing L, Wang C, Zhang Y, Yang X (2016) Recanalization, regrowth, and delayed rupture of a previously coiled unruptured anterior communicating artery aneurysm: a longitudinal hemodynamic analysis. World Neurosurg 89:726.e725–726.e710. https://doi.org/10.1016/j.wneu.2016.01.002
Longo M, Granata F, Racchiusa S, Mormina E, Grasso G, Longo GM, Garufi G, Salpietro FM, Alafaci C (2017) Role of hemodynamic forces in unruptured intracranial aneurysms: an overview of a complex scenario. World Neurosurg 105:632–642
Lorensen WE, Cline HE (1987) Marching cubes: a high resolution 3D surface construction algorithm. ACM Siggraph Comput Graph 21:163–169
Ma D, Tremmel M, Paluch RA, Levy EI, Meng H, Mocco J (2010) Size ratio for clinical assessment of intracranial aneurysm rupture risk. Neurol Res 32:482–486. https://doi.org/10.1179/016164109X12581096796558
Madhavan S, Kemmerling EMC (2018) The effect of inlet and outlet boundary conditions in image-based CFD modeling of aortic flow. Biomed Eng Online 17:66–66. https://doi.org/10.1186/s12938-018-0497-1
Meng H, Tutino V, Xiang J, Siddiqui A (2014) High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. Am J Neuroradiol 35:1254–1262
Miura Y, Ishida F, Umeda Y, Tanemura H, Suzuki H, Matsushima S, Shimosaka S, Taki W (2013) Low wall shear stress is independently associated with the rupture status of middle cerebral artery aneurysms. Stroke 44:519–521
Mocco J, Brown RD Jr, Torner JC, Capuano AW, Fargen KM, Raghavan ML, Piepgras DG, Meissner I, Huston J III, Investigators ISoUIA (2017) Aneurysm morphology and prediction of rupture: an international study of unruptured intracranial aneurysms analysis. Neurosurgery 82:491–496
Molyneux A, Kerr R, Group ISATC (2002) International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. J Stroke Cerebrovasc Dis 11:304–314
Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS (2015) The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 385:691–697
Piccinelli M, Steinman DA, Hoi Y et al (2012) Automatic neck plane detection and 3D geometric characterization of aneurysmal sacs. Ann Biomed Eng 40:2188–2211. https://doi.org/10.1007/s10439-012-0577-5
Qin H, Yang Q, Zhuang Q, Long J, Yang F, Zhang H (2017) Morphological and hemodynamic parameters for middle cerebral artery bifurcation aneurysm rupture risk assessment. J Korean Neurosurg Soc 60:504
Robertson AM, Duan X, Aziz KM, Hill MR, Watkins SC, Cebral JR (2015) Diversity in the strength and structure of unruptured cerebral aneurysms. Ann Biomed Eng 43:1502–1515. https://doi.org/10.1007/s10439-015-1252-4
Rodríguez-Pérez R, Vogt M, Bajorath JR (2017) Influence of varying training set composition and size on support vector machine-based prediction of active compounds. J Chem Inf Model 57:710–716
Schneiders JJ, Marquering HA, van den Berg R, VanBavel E, Velthuis B, Rinkel GJE, Majoie CB (2014) Rupture-associated changes of cerebral aneurysm geometry: high-resolution 3D imaging before and after rupture. Am J Neuroradiol 35:1358–1362. https://doi.org/10.3174/ajnr.A3866
Siogkas P, Sakellarios A, Exarchos T, Stefanou K, Fotiadis D, Naka K, Michalis L, Filipovic N, Parodi O (2011) Blood flow in arterial segments: rigid vs. deformable walls simulations. 5
Skodvin TØ, Johnsen L-H, Gjertsen Ø, Isaksen JG, Sorteberg A (2017) Cerebral aneurysm morphology before and after rupture. Stroke 48:880–886. https://doi.org/10.1161/STROKEAHA.116.015288
Smola AJ, Schölkopf B (2004) A tutorial on support vector regression. Stat Comput 14:199–222. https://doi.org/10.1023/b:stco.0000035301.49549.88
Sunderland K, Haferman C, Chintalapani G, Jiang J (2016) Vortex analysis of intra-aneurismal flow in cerebral aneurysms. Comput Math Methods Med 2016:16. https://doi.org/10.1155/2016/7406215
Sunderland K, Huang Q, Strother C, Jiang J (2019) Two closely spaced aneurysms of the supraclinoid internal carotid artery: how does one influence the other? J Biomech Eng 141. https://doi.org/10.1115/1.4043868
Sunderland K, Jiang J (2019) Multivariate analysis of hemodynamic parameters on intracranial aneurysm initiation of the internal carotid artery. Med Eng Phys. https://doi.org/10.1016/j.medengphy.2019.09.010
Tupin S, Saqr K, Ohta M (2020) Effects of wall compliance on multiharmonic pulsatile flow in idealized cerebral aneurysm models: comparative PIV experiments. Exp Fluids:61. https://doi.org/10.1007/s00348-020-02998-4
Varble N, Trylesinski G, Xiang J, Snyder K, Meng H (2017) Identification of vortex structures in a cohort of 204 intracranial aneurysms. J R Soc Interface 14:20170021. https://doi.org/10.1098/rsif.2017.0021
Varble N, Tutino VM, Yu J, Sonig A, Siddiqui AH, Davies JM, Meng H (2018) Shared and distinct rupture discriminants of small and large intracranial aneurysms. Stroke 49:856–864. https://doi.org/10.1161/STROKEAHA.117.019929
Vlak MH, Algra A, Brandenburg R, Rinkel GJ (2011) Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 10:626–636
Wang G-x, Wang S, L-l L, M-f G, Zhang D, Yang C-y, Wen L (2019) A simple scoring model for prediction of rupture risk of anterior communicating artery aneurysms. Front Neurol 10. https://doi.org/10.3389/fneur.2019.00520
Wang G-x, Zhang D, Wang Z-p, Yang L-q, Zhang L, Wen L (2016) Risk factors for the rupture of bifurcation intracranial aneurysms using CT angiography. Yonsei Med J 57:1178. https://doi.org/10.3349/ymj.2016.57.5.1178
Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ (2007) Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke 38:1404–1410
Xiang J, Natarajan SK, Tremmel M, Ma D, Mocco J, Hopkins LN, Siddiqui AH, Levy EI, Meng H (2011) Hemodynamic–morphologic discriminants for intracranial aneurysm rupture. Stroke 42:144–152
Zhang Y, Jing L, Liu J, Li C, Fan J, Wang S, Li H, Yang X (2016) Clinical, morphological, and hemodynamic independent characteristic factors for rupture of posterior communicating artery aneurysms. J NeuroInterv Surg 8:808–812. https://doi.org/10.1136/neurintsurg-2015-011865
Zhang Y, Jing L, Zhang Y, Liu J, Yang X (2016) Low wall shear stress is associated with the rupture of intracranial aneurysm with known rupture point: case report and literature review. BMC Neurol 16:231
Funding
The study was partially funded by a research contract through Siemens Medical Solution (USA) Inc. (Jiang), a pre-doctoral fellowship from the American Heart Association (18PRE33990321; Kevin Sunderland), a grant from the National Key Research and Development Program of China (2016YFC1300703; Qinghai Huang), and funding (cross-cutting initiatives) from the College of Engineering at the Michigan Technological University (Jiang).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and informed consent
The Institutional Review Board at the Michigan Technological University approved this study. This is a secondary and retrospective analysis of existing de-identified data. For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Neurosurgery - Aneurysm
Appendix
Appendix
A computational method based on informational entropy determined the spatially varying direction of the velocity field as a means to identify flow vortices. First, the 3D angular space of the flow velocity field was divided into 360 bins of equal area [25], resulting in cones connecting a unit sphere center and surface patches. Velocity vectors were then designated to a patch when said vector was contained within the connected cone. Assessing the local flow direction (x ϵ (x1, x2, x3,…xn)) of the velocity field (X), the probability p(xi) of flow direction can be used to calculate Shannon’s entropy.
Entropy is seen as minimal as the velocity directions concentrate toward one bin value and tend toward the maximum as the probability of velocity vectors in all directions becomes equally likely. Dividing H(X) by the maximum possible Shannon’s entropy (log2(N)) normalizes the entropy (NE) values between 0 and 1. For this work, a fixed volume of interest (VOI) was selected around each voxel within an IA: Nx × Ny × Nz; Nx = Ny = Nz = 11. Velocity vectors within each VOI, centered at each ith voxel, were used to determine the normalized entropy of each point in the flow field.
Reliance on normalized Shannon’s entropy to identify areas of flow vortices carries with it an inherent limitation: the inability to distinguish rotational flow vortices from random Brownian flow. From the perspective of Shannon’s entropy, both Brownian and rotational flow fields have a high probability that their velocity vectors will orient in any direction. The adaptation of aspects of the λ2 method of critical point analysis improves the NE’s ability to assess areas of vortices by identifying the central-most region (critical point) of swirling flow, with Brownian flow unlikely to have a centralized critical point [15]. The λ2 method decomposes the \( \mathbf{\nabla}\overrightarrow{\boldsymbol{v}} \) tensor into its strain-rate (symmetric: S) and spin (asymmetric: Ω) tensor:
The critical point of swirling flow is identified where S2 + Ω2 has two negative eigenvalues and λ1 > λ2 > λ3. When a proper point is identified, the dot product of the normalized velocity vector and eigenvector is calculated, relating the angle between the velocity vector and the degree of change of said vector: 0 as co-directional and 1 as orthogonal vectors.
The du(θ) value multiplied to NE to help distinguish swirling flow patterns from Brownian patterns: swirling patterns having lower values than Brownian patterns:
This combined method (CM) was used across all voxels within an IA dome for each time step of the final simulated cardiac cycle.
Once the CM was applied to all voxels within the 3D velocity field, the classic marching cube algorithm extracted the regions of vortex flow and mapped them to iso-surfaces [29]. To standardize the identification and extraction of vortex iso-surfaces, a set value > 0.3 of the combined method was used as the marching cube threshold value for all cases. To reduce the appearance of small, isolated areas of disturbed flow being mistaken for vortex flow, iso-surfaces with a volume < 0.5 mm3 were excluded from the analysis.
Rights and permissions
About this article
Cite this article
Sunderland, K., Wang, M., Pandey, A.S. et al. Quantitative analysis of flow vortices: differentiation of unruptured and ruptured medium-sized middle cerebral artery aneurysms. Acta Neurochir 163, 2339–2349 (2021). https://doi.org/10.1007/s00701-020-04616-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04616-y