Abstract
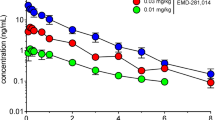
A central problem in the treatment of Parkinson’s disease (PD) is the development of motor disturbances like l-DOPA-induced dyskinesia (LID) after long-term treatment. Preclinical and clinical studies demonstrated that serotonin 5-HT1A receptor agonists attenuate this disabling motor side effect. The aim of this study was to investigate the ability of flibanserin compared to buspirone to attenuate l-DOPA-sensitized contraversive circling in hemiparkinsonian rats, which is an animal model of LID. Both drugs have a preferential affinity for the serotonin 5-HT1A receptors. Buspirone was in comparison because it was expected to have an effect in this model. Unilaterally 6-hydroxydopamine lesioned rats were treated twice daily intraperitoneally (ip) with l-DOPA methylester (12.5 mg/kg) and benserazide (3.25 mg/kg) for 21 days (on days 1, 3, 5, 8, 11, 14, 17 and 21). On day 24, l-DOPA-sensitized rats were treated ip 5 min prior to administration of l-DOPA methyl ester and benserazide with either saline (controls), 2.5, 5 and 10 mg/kg buspirone or flibanserin. Acute administration of both flibanserin and buspirone, dose dependently, attenuated the increased contraversive circling. An almost complete inhibition of the turning response was observed at 5 mg/kg buspirone and 10 mg/kg flibanserin. The current preclinical findings further implicate the 5-HT1A receptor as a promising therapeutic target for the reduction of LID and predict a potential efficacy of flibanserin in the treatment of LID in PD.

Similar content being viewed by others
Abbreviations
- AIMs:
-
Abnormal involuntary movements
- ip:
-
Intraperitoneally
- l-DOPA:
-
Levodopa, l-3,4-dihydroxyphenylalanine
- LID:
-
l-DOPA-induced dyskinesia
- PD:
-
Parkinson’s disease
- 5-HT:
-
Serotonin, 5-hydroxytryptamine
- 6-OHDA:
-
6-Hydroxydopamine
References
Apter JT, Allen LA (1999) Buspirone: future directions. J Clin Psychopharmacol 19:86–93
Arai R, Horiike K, Hasegawa Y (1998) Dopamine-degrading activity of monoamine oxidase in locus coeruleus and dorsal raphe nucleus neurons. A histochemical study in the rat. Neurosci Lett 250:41–44
Bibbiani F, Oh JD, Chase TN (2001) Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurology 57:1829–1834
Bonifati V, Fabrizio E, Cipriani R, Vanacore N, Meco G (1994) Buspirone in levodopa-induced dyskinesias. Clin Neuropharmacol 17:73–82
Borsini F, Evans K, Jason K, Rohde F, Alexander B, Pollentier S (2002) Pharmacology of flibanserin. CNS Drug Rev 8:117–142
Brotchie JM, Lee J, Venderova K (2005) Levodopa-induced dyskinesia in Parkinson’s disease. J Neural Transm 112:359–391
Carey RJ (1986) Relationship of changes in spontaneous motor activity to spontaneous circling in rats with unilateral 6-hydroxydopamine lesions. Exp Neurol 92:591–600
Carey RJ (1991) Chronic l-dopa treatment in the unilateral 6-OHDA rat-evidence for behavioral sensitization and biochemical tolerance. Brain Res 568:205–214
Carta M, Carlsson M, Kirik D, Björklund A (2007) Dopamine released from 5-HT terminals is the cause of l-dopa-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833
Cenci MA (2007) Dopamine dysregulation of movement control in l-DOPA-induced dyskinesia. Trends Neurosci 30:236–243
Cenci MA, Lee CS, Björklund A (1998) l-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci 10:2694–2706
Cooper DR, Marrel C, Testa B, van de Waterbeemd H, Quinn N, Jenner P, Marsden CD (1984) l-Dopa methyl ester: a candidate for chronic systemic delivery of l-Dopa in Parkinson’s disease. Clin Neuropharmacol 7:89–98
Dekundy A, Lundblad M, Danysz W, Cenci MA (2007) Modulation of l-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res 179:76–89
Dupre KB, Eskow KL, Negron B, Bishop C (2007) The differential effects of 5-HT(1A) receptor stimulation on dopamine receptor-mediated abnormal involuntary movements and rotations in the primed hemiparkinsonian rats. Brain Res 1158:135–143
Eskow KL, Gupta V, Alam S, Park JY, Bishop C (2007) The partial 5-HT1A agonist buspirone reduces the expression and delopment of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav 87:306–314
Eskow KL, Dupre KB, Barnum CJ, Dickinson SO, Park JY, Bishop C (2009) The role of the dorsal raphe nucleus in the development, expression, and treatment of l-dopa-induced dyskinesia in hemiparkinsonian rats. Synapse 63:610–620
Fox SH, Lang AE, Brotchie JM (2006) Translation of nondopaminergic treatments for levodopa-induced dyskinesia from MPTP-lesioned nonhuman primates to phase Ila clinical studies: keys to success and roads to failure. Mov Disord 21:1578–1594
Gerlach M, Riederer P (1996) Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm 103:987–1041
Gerlach M, van den Buuse M, Blaha C, Bremen D, Riederer P (2004) Entacapone increases and prolongs the central effects of l-DOPA in the 6-hydroxydopamine-lesioned rat. Naunyn Schmiedeberg′s Arch Pharmacol 370:388–394
Henry B, Crossman AR, Brotchie JM (1999) Effect of repeated l-DOPA, bromocriptine, or lisuride administration on preproenkephalin-A and preproenkephalin-B mRNA levels in the striatum of the 6-hydroxydopamine-lesioned rat. Exp Neurol 155:204–220
Iravani MM, Jackson MJ, Kuoppamaki M, Smith LA, Jenner P (2003) 3, 4-methylenedioxymethamphetamine (ecstasy) inhibits dyskinesia expression and normalizes motor activity in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-treated primates. J Neurosci 23:9107–9115
Jankovic JJ (2005) Motor fluctuations and dyskinesias in Parkinson’s disease: clinical manifestations. Mov Disord 20(Suppl. 11):S11–S16
Jenner P (2008) Molecular mechanisms of l-DOPA-induced dyskinesia. Nat Rev Neurosci 9:665–677
Lane EL, Cheetham SC, Jenner P (2006) Does contraversive circling in the 6-OHDA-lesioned rat indicate an ability to induce motor complications as well as therapeutic effects in Parkinson’s disease? Exp Neurol 197:284–290
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15:120–132
Lundblad M, Picconi B, Lindgren H, Cenci MA (2004) A model of l-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis 16:110–123
McCall RB, Romero AG, Bienkowski MJ, Harris DW, McGuire JC, Piercey MF, Shuck ME, Smith MW, Svensson KA, Schreur PJKD, Carlsson A, Vonvoigtlander PF (1994) Characterization of U-92016A as a selective, orally active, high intrinsic activity 5-hydroxytryptamine1A agonist. J Pharmacol Exp Ther 271:875–883
McMillen BA, Matthews RT, Sanghera MK, Shepard PD, German DC (1983) Dopamine receptor antagonism by the novel anti-anxiety drug, buspirone. J Neurosci 3:733–738
Müller T, Russ H (2006) Levodopa, motor fluctuations and dyskinesia in Parkinson’s disease. Expert Opin Pharmacol 7:1715–1730
Müller T, Woitalla D, Russ H, Hock K, Haeger DA (2007) Prevalence and treatment strategies of dyskinesia in patients with Parkinson’s disease. J Neural Transm 114:1023–1026
Nadjar A, Gerfen CR, Bezard E (2009) Priming for l-dopa-induced dyskinesia in Parkinson’s disease: a feature inherent to the treatment or the disease? Prog Neurobiol 87:1–9
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, San Diego
Rylander D, Parent M, O’Sullivan SS, Dovero S, Lees AJ, Bezard E, Descarries L, Cenci MA (2010) Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann Neurol 68:619–628
Schwarz J, Strecker K, Ohm S, Beck J (2005) Flibanserin but not buspirone reduces levodopa-induced dyskinesia in RGS9 deficient mice. J Neurol Sci 238(Suppl. 1):369
Taylor DP (1988) Buspirone, a new approach to the treatment of anxiety. FASEB J 2:2445–2452
Ungerstedt U, Arburthnott GW (1970) Quantitative recording of rotational behaviour in rats after 6-hydroxydopamine lesions in the nigrostriatal dopamine system. Brain Res 24:485–493
Zeng BY, Iravani MM, Jackson MJ, Rose S, Parent A, Jenner P (2010) Morphological changes in serotoninergic neurites in the striatum and globus pallidus in levodopa primed MPTP treated common marmosets with dyskinesia. Neurobiol Dis 40:599–607
Acknowledgments
This study was funded by Monitoring Force GmbH (Münster, Germany) without any restriction.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerlach, M., Beck, J., Riederer, P. et al. Flibanserin attenuates l-DOPA-sensitized contraversive circling in the unilaterally 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. J Neural Transm 118, 1727–1732 (2011). https://doi.org/10.1007/s00702-010-0570-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-010-0570-9