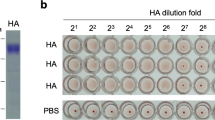
Abstract
Monitoring avian influenza (AI) infection and detecting silent infection in vaccinated chickens has been challenging due to the lack of effective serological diagnostic assays to differentiate between vaccinated and infected animals. Very few studies have identified suitable proteins in AI virus that can be used in successfully differentiating infected from vaccinated animals (DIVA). An HA2 peptide: HA2 position 197-201 (HA position 488-516) described by Khurana et al. (J Virol 85(23):12455–12463, 2011), was shown to have DIVA ability by differentiating H5N1-infected human sera in ELISA. In order to explore the capacity of the HA2 protein, as a DIVA reagent in chickens, four overlapping recombinant HA2 proteins, were expressed in E. coli and tested for reaction with H5N1 sera obtained from infected and vaccinated chickens. Recombinant protein HA2_B2 (380-461) was able to generate a detectable reaction with both H5N1 infected and vaccinated chicken sera but recombinant protein HA2_B4 (483-565) reacted strongly only with sera obtained from chickens infected with live virus, confirming its suitability as a DIVA antigen. Further analysis of the HA2 using several overlapping peptides suggested that positions 380-461 and 483-565 were antigenic in mouse and chicken. This study, for the first time, identified novel antigenic epitopes on the H5N1 HA2 subunit. Two epitopes, found in the HA2 ectodomain, have never been described for AIV infection in any animal species. Also one HA2 epitope was found to have high potential as a DIVA antigen.









Similar content being viewed by others
References
Claas E, Osterhaus A, van Beek RDJJ, Rimmelzwaan G, Senne D, Krauss S, Shortridge K, Webster R (1998) Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet (London, England) 351(9111):472–477
Georgiev VS (2009) Influenza. In: Georgiev VS (ed) National Institute of Allergy and Infectious Diseases, NIH, vol 2. Humana Press, New York, pp 85–102. https://doi.org/10.1007/978-1-60327-297-1_13
World Organisation for Animal Health (OIE) (2004) Disease information. vol 17. No 6. OIE, Paris. ftp://ftp.oie.int/infos_san_archives/eng/2004/en_040206v17n06.pdf. Accessed 15 Feb 2012
Suarez DL, Schultz-Cherry S (2000) Immunology of avian influenza virus: a review. Dev Comp Immunol 24(2–3):269–283. https://doi.org/10.1016/S0145-305X(99)00078-6
Lee CW, Saif Y (2009) Avian influenza virus. Comp Immunol Microbiol Infect Dis 32(4):301–310
Swayne DE (2009) Avian influenza vaccines and therapies for poultry. Comp Immunol Microbiol Infect Dis 32(4):351–363. https://doi.org/10.1016/j.cimid.2008.01.006
Siregar ES, Darminto J, Weaver A, Bouma A (2007) The vaccination programme in Indonesia. Dev Biol 130:151–158
Capua I, Cattoli G, Marangon S (2004) DIVA-a vaccination strategy enabling the detection of field exposure to avian influenza. Dev Biol 119:229–233
Hulst M, Westra D, Wensvoor TG, Moormann R (1993) Glycoprotein E1 of hog cholera virus expressed in insect cells protects swine from hog cholera. J Virol 67(9):5435–5442
van Oirschot JT (1999) DIVA vaccines that reduce virus transmission. J Biotechnol 73(2–3):195–205
Oirschot JTV, Gielkensa ALJ, Moormanna RJM, Berns AJM (1990) Marker vaccines, virus protein-specific antibody assays and the control of Aujeszky’s disease. Vet Microbiol 23(1–4):85–101. https://doi.org/10.1016/0378-1135(90)90139-M
Neitzert E, Beck E, Mello PAd, Gomes I, Bergmann IE (1991) Expression of the aphthovirus rna polymerase gene in Escherichia coli and its use together with other bioengineered nonstructural antigens in detection of late persistent infections. Virology 184(2):799–804
Capua I, Terregino C, Cattoli G, Mutinelli F, Rodriguez JF (2003) Development of a DIVA (Differentiating Infected from Vaccinated Animals) strategy using a vaccine containing a heterologous neuraminidase for the control of avian influenza. Avian Pathol 32(1):47–55. https://doi.org/10.1080/0307945021000070714
Cattoli G, Milani A, Bettini F, Serena Beato M, Mancin M, Terregino C, Capua I (2006) Development and validation of an anti-N3 indirect immunofluorescent antibody test to be used as a companion diagnostic test in the framework of a “DIVA” vaccination strategy for avian influenza infections in poultry. Avian Pathol 35(2):154–159. https://doi.org/10.1080/03079450600598079
Kim M-C, Jeong O-M, Kang H-M, Song C-S, Kwon J-H, Lee Y-J (2009) Novel use of a N2-specific enzyme-linked immunosorbent assay for differentiation of infected from vaccinated animals (DIVA)-based identification of avian influenza. Vaccine 27(24):3189–3194
Avellaneda G, Mundt E, Lee C-W, Jadhao S, Suarez DL (2010) Differentiation of infected and vaccinated animals (DIVA) using the NS1 protein of avian influenza virus. Avian Dis 54(s1):278–286. https://doi.org/10.1637/8644-020409-reg.1
Hadifar F, Ignjatovic J, Tarigan S, Indriani R, Ebrahimie E, Hasan NH, McWhorter A, Putland S, Ownagh A, Hemmatzadeh F (2014) Multimeric recombinant M2e protein-based ELISA: a significant improvement in differentiating avian influenza infected chickens from vaccinated ones. PloS One 9(10):e108420. https://doi.org/10.1371/journal.pone.0108420
Hemmatzadeh F, Sumarningsih Tarigan S, Indriani R, Dharmayanti NL, Ebrahimie E, Ignjatovic J (2013) Recombinant M2e protein-based ELISA: a novel and inexpensive approach for differentiating avian influenza infected chickens from vaccinated ones. PloS One 8(2):e56801. https://doi.org/10.1371/journal.pone.0056801
Kim M-C, Choi J-G, Kwon J-S, Kang H-M, Paek M-R, Jeong O-M, Kwon J-H, Lee Y-J (2010) Field application of the H9M2e enzyme-linked immunosorbent assay for differentiation of H9N2 avian influenza virus-infected chickens from vaccinated chickens. Clin Vaccine Immunol 17(12):1977–1984
Victor B, Victor A, Baptista C, Soares CM (2012) Structural determinants for the membrane insertion of the transmembrane peptide of hemagglutinin from influenza virus. J Chem Inf Model 52(11):3001–3012
Varečková E, Mucha V, Kostolanský F (2013) HA2 glycopolypeptide of influenza A virus and antiviral immunity. Acta Virol 57:247–256
Chen J, Wharton SA, Weissenhorn W, Calder LJ, Hughson FM, Skehel JJ, Wiley DC (1995) A soluble domain of the membrane-anchoring chain of influenza virus hemagglutinin (HA2) folds in Escherichia coli into the low-pH-induced conformation. Proc Natl Acad Sci USA 92(26):12205–12209
Varečková E, Mucha V, Kostolanský F, Gubareva L, Klimov A (2008) HA2-specific monoclonal antibodies as tools for differential recognition of influenza A virus antigenic subtypes. Virus Res 132(1–2):181–186
Shibo J, Lu L, Qi L, Wei X, Lanying D (2012) Receptor-binding domains of spike proteins of emerging or re-emerging viruses as targets for development of antiviral vaccines. Emerg Microbes Infect 1(8):e13. https://doi.org/10.1038/emi.2012.1
Bommakanti G, Citron M, Hepler R, Callahan C, Heidecker G, Najar T, Lu X, Joyce J, Shiver J, Casimiro D, ter Meulen J, Liang X, Varadarajan R (2010) Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc Natl Acad Sci USA 107(31):13701–13706
Mueller M, Renzullo S, Brooks R, Ruggli N, Hofmann MA (2010) Antigenic characterization of recombinant hemagglutinin proteins derived from different avian influenza virus subtypes. PloS One 5(2):e9097. https://doi.org/10.1371/journal.pone.0009097
Nobusawa E, Aoyama T, Kato H, Suzuki Y, Tateno Y, Nakajima K (1991) Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology 182(2):475–485. https://doi.org/10.1016/0042-6822(91)90588-3
Gerhard W, Mozdzanowska K, Zharikova D (2006) Prospects for universal influenza virus vaccine. Emerg Infect Dis 12(4):569–574
Fan X, Hashem AM, Chen Z, Li C, Doyle T, Zhang Y, Yi Y, Farnsworth A, Xu K, Li Z, He R, Li X, Wang J (2015) Targeting the HA2 subunit of influenza A virus hemagglutinin via CD40L provides universal protection against diverse subtypes. Mucosal Immunol 8(1):211–220. https://doi.org/10.1038/mi.2014.59
Ekiert DC, Wilson IA (2012) Broadly neutralizing antibodies against influenza virus and prospects for universal therapies. Curr Opin Virol 2(2):134–141. https://doi.org/10.1016/j.coviro.2012.02.005
Gocník M, Fislová T, Sládková T, Mucha V, Kostolanský F, Varečková E (2007) Antibodies specific to the HA2 glycopolypeptide of influenza A virus haemagglutinin with fusion-inhibition activity contribute to the protection of mice against lethal infection. J Gen Virol 88(3):951–955. https://doi.org/10.1099/vir.0.82563-0
Styk B, Russ G, Polakova K (1979) Antigenic glycopolypeptides HA1 and HA2 of influenza virus haemagglutinin. III. Reactivity with human convalescent sera. Acta Virol 23(1):1–8
Kostolanský F, Mucha V, Slováková R, Varečková E (2002) Natural influenza A virus infection of mice elicits strong antibody response to HA2 glycopolypeptide. Acta Virol 46(4):229–236
Becht H, Huang RT, Fleischer B, Boschek CB, Rott R (1984) Immunogenic properties of the small chain HA2 of the haemagglutinin of influenza viruses. J Gen Virol 65(1):173–183
Russ G, Polakova K, Kostolanska F, Styk B, Vancakova M (1987) Monoclonal antibodies to glycopolypeptides HA1 and HA2 of influenza virus haemagglutinin. Acta Virol 31(5):374–386
Sánchez Fauquier A, Villanueva N, Melero JA (1987) Isolation of cross-reactive, subtype-specific monoclonal antibodies against influenza virus HA1 and HA2 hemagglutinin subunits. Arch Virol 97(3–4):251–265
Varečková E, Wharton SA, Mucha V, Gocník M, Kostolanský F (2003) A monoclonal antibody specific to the HA2 glycoprotein of influenza A virus hemagglutinin that inhibits its fusion activity reduces replication of the virus. Acta Virol 47(4):229–236
Fislová T, Sládková T, Gocník M, Mucha V, Varečková E, Kostolanský F (2005) Differences in antibody responses of mice to intranasal or intraperitoneal immunization with influenza A virus and vaccination with subunit influenza vaccine. Acta Virol 49(4):243–250
Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P (2012) Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol 86(10):5774–5781. https://doi.org/10.1128/jvi.00137-12
Yen HL, Peiris JSM (2009) Mapping antibody epitopes of the avian H5N1 influenza virus. PLoS Med 6(4):e1000064. https://doi.org/10.1371/journal.pmed.1000064
Bommakanti G, Lu X, Citron M, Najar T, Heidecker G, ter Meulen J, Varadarajan R, Liang X (2012) Design of Escherichia coli-expressed stalk domain immunogens of H1N1 hemagglutinin that protect mice from lethal challenge. J Virol 86(24):13434–13444
Chambers TM, Kawaoka Y, Webster RG (1988) Protection of chickens from lethal influenza infection by vaccinia-expressed hemagglutinin. Virology 167(2):414–421
Chee Wei T, Nurul Wahida AG, Shaharum S (2014) Construction and heterologous expression of a truncated haemagglutinin (HA) protein from the avian influenza virus H5N1 in Escherichia coli. Trop Biomed 31(4):1–10
Janulíková J, Staneková Z, Mucha V, Kostolanský F, Varečková E (2012) Two distinct regions of HA2 glycopolypeptide of influenza virus hemagglutinin elicit cross-protective immunity against influenza. Acta Virol 56(3):169–176
Halliley JL, Khurana S, Krammer F, Fitzgerald T, Coyle EM, Chung KY, Baker SF, Yang H, Martinez-Sobrido L, Treanor JJ, Subbarao K, Golding H, Topham DJ, Sangster MY (2015) High affinity H7 head and stalk domain-specific antibody responses to an inactivated influenza H7N7 vaccine after priming with live attenuated influenza vaccine. J Infect Dis. https://doi.org/10.1093/infdis/jiv210
Wohlbold TJ, Hirsh A, Krammer F (2015) An H10N8 influenza virus vaccine strain and mouse challenge model based on the human isolate A/Jiangxi-Donghu/346/13. Vaccine 33(9):1102–1106. https://doi.org/10.1016/j.vaccine.2015.01.026
Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, García-Sastre A, Palese P (2012) Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci 109(7):2573–2578. https://doi.org/10.1073/pnas.1200039109
Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, García-Sastre A, Palese P, Treanor JJ, Krammer F (2013) H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol 87(8):4728–4737. https://doi.org/10.1128/jvi.03509-12
Armstrong RT, Kushnir AS, White JM (2000) The transmembrane domain of influenza hemagglutinin exhibits a stringent length requirement to support the hemifusion to fusion transition. J Cell Biol 151(2):425–438. https://doi.org/10.1083/jcb.151.2.425
Doyle C, Sambrook J, Gething MJ (1986) Analysis of progressive deletions of the transmembrane and cytoplasmic domains of influenza hemagglutinin. J Cell Biol 103(4):1193–1204
Steel J, Lowen A, Wang T, Yondola M, Gao Q, Haye K, García-Sastre A, Palese P (2010) Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 1(1):e00018-00010
Lu Y, Welsh JP, Swartz JR (2014) Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc Natl Acad Sci 111(1):125–130. https://doi.org/10.1073/pnas.1308701110
Swalley S, Baker B, Calder L, Harrison S, Skehel J, Wiley D (2004) Full-length influenza hemagglutinin HA2 refolds into the trimeric low-pH-induced conformation. Biochemistry 43(19):5902–5911
Okuno Y, Isegawa Y, Sasao F, Ueda S (1993) A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J Virol 67(5):2552–2558
Sui J, Hwang W, Perez S, Wei G, Aird D, Lm Chen, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox N, Bankston L, Donis R, Liddington R, Marasco W (2009) Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 16(3):265–273
Varečková E, Mucha V, Wharton SA, Kostolanský F (2003) Inhibition of fusion activity of influenza A haemagglutinin mediated by HA2-specific monoclonal antibodies. Arch Virol 148(3):469–486
Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, Palese P (2010) Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog 6(2):e1000796. https://doi.org/10.1371/journal.ppat.1000796
Khurana S, Sasono P, Fox A, Nguyen V, Le Q, Pham Q, Nguyen T, Horby P, Golding H (2011) H5N1-SeroDetect EIA and rapid test: a novel differential diagnostic assay for serodiagnosis of H5N1 infections and surveillance. J Virol 85(23):12455–12463
Han X, Bushweller JH, Cafiso DS, Tamm LK (2001) Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat Struct Biol 8(8):715–720
Becker WB, Uys CJ (1967) Experimental infection of chickens with influenza A/Tern/South Africa/1961 and Chicken/Scotland/1959 viruses: I. Clinical picture and virology. J Comp Pathol 77(2):159–165. https://doi.org/10.1016/0021-9975(67)90006-0
Dybing JK, Schultz Cherry S, Swayne DE, Suarez DL, Perdue ML (2000) Distinct pathogenesis of Hong Kong-origin H5N1 viruses in mice compared to that of other highly pathogenic H5 avian influenza viruses. J Virol 74(3):1443–1450
De BK, Shaw MW, Rota PA, Harmon MW, Esposito JJ, Rott R, Cox NJ, Kendal AP (1988) Protection against virulent H5 avian influenza virus infection in chickens by an inactivated vaccine produced with recombinant vaccinia virus. Vaccine 6(3):257–261. https://doi.org/10.1016/0264-410X(88)90221-6
Bonnafous P, Nicolaï M-C, Taveau J-C, Chevalier M, Barrière F, Medina J, Le Bihan O, Adam O, Ronzon F, Lambert O (2014) Treatment of influenza virus with beta-propiolactone alters viral membrane fusion. Biochimica et Biophysica Acta Biomembranes 1838 1, Part B:355–363. https://doi.org/10.1016/j.bbamem.2013.09.021
Hashem A, Van Domselaar G, Li C, Wang J, She Y-M, Cyr T, Sui J, He R, Marasco W, Li X (2010) Universal antibodies against the highly conserved influenza fusion peptide cross-neutralize several subtypes of influenza A virus. Biochem Biophys Res Commun 403(2):247–251
Kim JI, Park M-S (2012) N-Linked glycosylation in the hemagglutinin of influenza A viruses. Yonsei Med J 53(5):886–893
Gopal GJ, Kumar A (2013) Strategies for the production of recombinant protein in Escherichia coli. Protein J 32(6):419–425. https://doi.org/10.1007/s10930-013-9502-5
Bosshard HR (1995) Epitope mapping with peptides. In: Merrifield BG (ed) Peptides: synthesis, structures and applications, Academic Press Inc, California, pp 419–454
Webster RG, Govorkova EA (2014) Continuing challenges in influenza. Ann N Y Acad Sci. https://doi.org/10.1111/nyas.12462
Choi W-S, Lloren KKS, Baek YH, Song M-S (2017) The significance of avian influenza virus mouse-adaptation and its application in characterizing the efficacy of new vaccines and therapeutic agents. Clin Exp Vaccine Res 6(2):83–94. https://doi.org/10.7774/cevr.2017.6.2.83
Swayne DE, Pavade G, Hamilton K, Vallat B, Miyagishima K (2011) Assessment of national strategies for control of high-pathogenicity avian influenza and low-pathogenicity notifiable avian influenza in poultry, with emphasis on vaccines and vaccination. Revue Scientifique et Technique Office international des Épizooties 30(3):839–870
Acknowledgements
Funding was provided by Australian Centre for International Agricultural Research (AU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Khrisdiana declares that she has no conflict of interest. Author Nadeeka declares that she has no conflict of interest. Author Jagoda declares that she has no conflict of interest. Author Amir declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the author.
Additional information
Handling Editor: Ayato Takada.
Rights and permissions
About this article
Cite this article
Putri, K., Wawegama, N., Ignjatovic, J. et al. Characterisation of the antigenic epitopes in the subunit 2 haemagglutinin of avian influenza virus H5N1. Arch Virol 163, 2199–2212 (2018). https://doi.org/10.1007/s00705-018-3896-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3896-5