Abstract
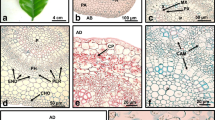
The objective of this study was to morpho-anatomically characterize embryogenic rice calli during early induction of somatic embryogenesis of three Brazilian rice cultivars. Herein, we explored embryogenic units (EUs) from 2-week-old cut proliferated calli to verify whether they were suitable for Agrobacterium tumefasciens-mediated transformation. Histological analysis and scanning electron microscopy (SEM) were used to analyze these types of calli during early rice callogenesis in the cultivars BRS Primavera, BRS Bonança, and BRS Caiapó. The characteristics of the embryogenic cells were preserved in the EUs, which showed a globular, compact structure that contained tightly packed cells and thus rendered the cells suitable for transformation. The EUs of BRS Caiapó also maintained the characteristics of the non-embryogenic callus, such as an elongated morphology and a lack of cellular organization. In general, the observations of the histological sections corresponded with those of the SEM images. The histological analysis suggested that all cultivars used in these experiments have morphogenic potential. The EUs from proliferated 2-week-old cut calli maintained their embryogenic features. The EUs were subjected to Agrobacterium-mediated transformation, which exhibited a regeneration frequency of 58 % for transformed hygromycin-resistant cell lines. These results show that EUs from proliferated 2-week-old cut calli are suitable for plant transformation.





Similar content being viewed by others
Abbreviations
- ECM:
-
Extracellular matrix
- ECMSN:
-
Extracellular matrix surface network
- EUs1:
-
Embryogenic units
- EUs2:
-
Proliferated 2-week-old cut calli
- IM:
-
Induction medium
- MS:
-
Murashige and Skoog
- SEM:
-
Scanning electron microscopy
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
References
Abe T, Futsuhara Y (1985) Efficient plant regeneration from protoplast through somatic embryogenesis. Biol Technol 4:1087–1090
Alfonso-Rubi J, Carbonero P, Diaz I (1999) Parameters influencing the regeneration capacity of calluses derived from mature indica and japonica rice seeds after microprojectile bombardment. Euphytica 107:115–122
Brisibe EA, Miyake H, Taniguchi T et al (1992) Callus formation and scanning electron microscopy of plantlet regeneration in African rice (Oryza glaberrima Steud). Plant Sci 83:217–224
Chapman A, Blervacq AS, Hendrix T et al (2000) Cell wall differentiation during early somatic embryogenesis in plants. II. Ultrastructural study and pectin immunolocalization on chicory embryos. Can J Bot 78:824–831
Chen TH, Lam L, Chen SC (1985) Somatic embryogenesis and plant regeneration from cultured young inflorescences of Oryza sativa L. (rice). Plant Cell Tissue Org Cult 4:51–54
Datta K, Tu JM, Oliva N et al (2001) Enhanced resistance to sheath blight by constitutive expression of infection-related rice chitinase in transgenic elite indica rice cultivars. Plant Sci 160:405–414
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Report 4:19–21
Dong J, Teng W, Buchholz WG et al (1996) Agrobacterium-mediated transformation of javanica rice. Mol Breed 2:267–276
Dubois T, Guedira M, Vasseur J (1991) Direct somatic embryogenesis in leaves of Cichorium. A histological and SEM study of early stages. Protoplasma 162:120–127
Fehér A, Pasternak T, Otvos K et al (2002) Induction of embryogenic competence in somatic plant cells: a review. Biologia 57:5–12
Hiei Y, Komari T (2008) Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat Protoc 3:824–834
Hiei Y, Ohta S, Komari T et al (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hiei Y, Ohta S, Komari T et al (1997) Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol Biol 35:205–218
Jefferson RA (1987) Assaying chimeric genes in plants: the Gus gene fusion system. Plant Mol Biol Report 5:387–405
Jones TJ, Rost TL (1989) The developmental anatomy and ultrastructural of somatic embryos from rice (Oryza sativa L.) scutellum epithelial cells. Bot Gaz 150:41–49
Kachar B, Parakkal M, Frex J (1990) Structural basis form mechanical transduction in the frog vestibular sensory apparatus: the otholitic membrane. Hear Res 45:179–190
Karnovsky MJ (1965) A formaldehyde–glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137–138
Khatun MM, Ali MH, Desamero NV (2003) Effect of genotype and culture media on callus formation and plant regeneration from mature seed scutella culture in rice. Plant Tissue Cult 13:99–107
Konieczny R, Bohdanowicz J, Czaplicki AZ et al (2005) Extracellular matrix surface network during plant regeneration in wheat anther culture. Plant Cell Tissue Org Cult 83:201–208
Lai KS, Yusoff K, Maziah M (2011) Extracellular matrix as the early structural marker for Centella asiatica embryogenic tissues. Biol Plant 55:549–553
Lee KS, Jeon HS, Kim MY (2002) Optimization of a mature embryo-based in vitro culture system for high-frequency somatic embryogenic callus induction and plant regeneration from japonica rice cultivars. Plant Cell Tissue Org Cult 71:237–244
Lin YJ, Zhang Q (2005) Optimizing the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep 23:540–547
McCann MC, Wells B, Roberts K (1990) Direct visualization of cross-links in the primary plant cell wall. J Cell Sci 96:323–334
Mendoza AB, Futsuhara Y (1992) Histological observations on plant regeneration in rice (Oryza sativa L.) calli. Jpn J Breed 42:33–41
Mendoza AB, Hattori K, Nishimura T et al (1993) Histological and scanning electron microscopic observations on plant regeneration in mungbean cotyledon (Vigna radiata (L.) Wilczek) cultured in vitro. Plant Cell Tissue Org Cult 32:137–143
Molina D, Aponte M, Cortina H (2002) The effect of genotype and explant age on somatic embryogenesis of coffee. Plant Cell Tissue Org Cult 71:117–123
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:437–497
Nabors MW, Heyser JW, Dykes TA et al (1983) Long-duration, high-frequency plant regeneration from cereal tissue cultures. Planta 157:385–391
Nakagawa Y, Machida C, Machida Y et al (2000) Frequency and pattern of transposition of the maize transposable element Ds in transgenic rice plants. Plant Cell Physiol 41:733–742
Nakamura T, Maeda E (1989) A scanning electron microscope study on japonica type rice callus cultures, with emphasis on plantlet initiation. Jpn J Crop Sci 58:395–403
Namasivayam P (2007) Acquisition of embryogenic competence during somatic embryogenesis. Plant Cell Tissue Org Cult 90:1–8
Namasivayam P, Skepper J, Hanke D (2006) Identification of a potential structural marker for embryogenic competency in the Brassica napus ssp. Oleifera embryogenic tissue. Plant Cell Rep 25:887–895
Narciso JO, Hattori K (2010) Genotypic differences in morphology and ultrastructures of callus derived from selected rice varieties. Philipp Sci Lett 3:59–65
Nishimura S, Maeda E (1977) Histological studies of callus induction in rice seed. Jpn J Crop Sci 46:275–285
Ovečka M, Bobák M, Blehová A et al (1998) Papaver somniferum regeneration by somatic embryogenesis and shoot organogenesis. Biol Plant 40:321–328
Pescador R, Araújo PS, Maas CH et al (2000) Biotecnologia de Piper hispidinervium—pimenta longa. Biotecnol Cienc Desenvolv 3:19–23
Pilarska M, Czaplicki A, Konieczny R (2007) Patterns of pectin epitope expression during shoot and root regeneration in androgenic cultures of two wheat cultivars. Acta Biol Cracov Bot 49:69–72
Popielarska M, Ślesak H, Goralski G (2006) Histological and SEM studies on organogenesis in endosperm-derived callus of kiwifruit (Actinidia deliciosa cv. hayward). Acta Biol Cracov Bot 48:97–104
Popielarska-Konieczna M, Bohdanowicz J, Starnawska E (2010) Extracellular matrix of plant callus tissue visualized by ESEM and SEM. Protoplasma 247:121–125
Popielarska-Konieczna M, Kozieradzka-Kiszkurno M, Bohdanowicz J (2011) Cutin plays a role in differentiation of endosperm-derived callus of kiwifruit. Plant Cell Rep 30:2143–2152
Pravin VJ, Dudhare MS, Saluja T, Sarawgi C (2011) Assessment of critical factors influencing callus induction, in vitro regeneration and selection of bombarded indica rice genotypes. J Agric Biotechnol Sustain Dev 3:44–59
Quiroz-Figueroa FR, Rojas-Herrera R, Galaz-Avalos RM et al (2006) Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tissue Org Cult 86:285–301
Rashid H, Bokhari SYA, Quraishi A (2001) Callus induction, regeneration and hygromycin selection of rice (Super Basmati). Online J Biol Sci 1:1145–1146
Saika H, Toki S (2010) Mature seed-derived callus of the model indica rice variety Kasalath is highly competent in Agrobacterium-mediated transformation. Plant Cell Rep 29:1351–1364
Sallaud C, Meynard D, Van Boxtel J et al (2003) Highly efficient production and characterization of T-DNA plants for rice (Oryza sativa L.) functional genomics. Theor Appl Genet 106:1396–1408
Samaj MB, Bobak M, Blehova A et al (1995) Developmental SEM observations on an extracellular matrix in embryogenic calli of Drosera rotundifolia and Zea mays. Protoplasma 186:45–49
Samaj J, Baluška F, Bobák M et al (1999) Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monoclonal antibody JIM4. Plant Cell Rep 18:369–374
Sangduen N, Klamsomboon P (2001) Histological and scanning electron observations on embryogenic and non-embryogenic calli of aromatic Thai rice (Oryza sativa L. cv. Khao Daw Mali 105). Kasetsart J (Nat Sci) 35:427–432
Silva PI Jr, Daffre S, Bulet P (2000) Isolation and characterization of gomesin, an 18-residue cysteine-rich defense peptide from the spider Acanthoscurria gomesiana hemocytes with sequence similarities to horseshoe crab antimicrobial peptides of the tachyplesin family. J Biol Chem 275:33464–33470
Vega R, Vásquez N, Espinoza AM et al (2009) Histology of somatic embryogenesis in rice (Oryza sativa cv. 5272). Rev Biol Trop 57:141–150
Verdeil JL, Hocher V, Huet C et al (2001) Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Ann Bot 88:9–18
Visarada KBRS, Sailaja M, Sarma NP (2002) Effect of callus induction media on morphology of embryogenic calli in rice genotypes. Biol Plant 45:495–502
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behaviour of cells as an embryogenic group. Ann Bot 57:443–462
Yusoff NFM, Alwee SSRS, Abdullah MO (2012) A time course anatomical analysis of callogenesis from young leaf explants of oil palm (Elaeis guineensis Jacq.). J Oil Palm Res 24:1330–1341
Acknowledgments
We thank the Universidade Federal de Goiás for the use of the LabMIC and the Laboratório de Morfologia e Anatomia de Plantas. This work was funded by Embrapa and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Pavla Binarova
Rights and permissions
About this article
Cite this article
Bevitori, R., Popielarska-Konieczna, M., dos Santos, E.M. et al. Morpho-anatomical characterization of mature embryo-derived callus of rice (Oryza sativa L.) suitable for transformation. Protoplasma 251, 545–554 (2014). https://doi.org/10.1007/s00709-013-0553-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-013-0553-4