Abstract
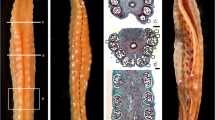
Brachypodium distachyon has emerged as a model plant for the improvement of grain crops such as wheat, barley and oats and for understanding basic biological processes to facilitate the development of grasses as superior energy crops. Brachypodium is also the first species of the grass subfamily Pooideae with a sequenced genome. For obtaining a better understanding of the mechanisms controlling male gametophyte development in B. distachyon, here we report the cellular changes during the stages of anther development, with special reference to the development of the anther wall. Brachypodium anthers are tetrasporangiate and follow the typical monocotyledonous-type anther wall formation pattern. Anther differentiation starts with the appearance of archesporial cells, which divide to generate primary parietal and primary sporogenous cells. The primary parietal cells form two secondary parietal layers. Later, the outer secondary parietal layer directly develops into the endothecium and the inner secondary parietal layer forms an outer middle layer and inner tapetum by periclinal division. The anther wall comprises an epidermis, endothecium, middle layer and the secretory-type tapetum. Major documented events of anther development include the degradation of a secretory-type tapetum and middle layer during the course of development and the rapid formation of U-shaped endothecial thickenings in the mature pollen grain stage. The tapetum undergoes degeneration at the tetrad stage and disintegrates completely at the bicellular stage of pollen development. The distribution of insoluble polysaccharides in the anther layers and connective tissue through progressive developmental stages suggests their role in the development of male gametophytes. Until sporogenous cell stage, the amount of insoluble polysaccharides in the anther wall was negligible. However, abundant levels of insoluble polysaccharides were observed during microspore mother cell and tetrad stages and gradually declined during the free microspore and vacuolated microspore stages to undetectable level at the mature stage. Thus, the cellular features in the development of anthers in B. distachyon share similarities with anther and pollen development of other members of Poaceae.











Similar content being viewed by others
References
Alves SC, Worland B, Thole V, Snape JW, Bewan MW, Vain P (2009) A protocol for Agrobacterium-mediated transformation of Brachypodium distachyon community standard line Bd21. Nat Protoc 4:638–649
Aybeke M (2012) Anther wall and pollen development in Ophrys mammosa L. (Orchidaceae). Plant Syst Evol 298:1015–1023
Bedinger PA (1992) The remarkable biology of pollen. Plant Cell 4:879–887
Bevan MW, Garvin DF, Vogel JP (2010) Brachypodium distachyon genomics for sustainable food and fuel production. Curr Opin Biotech 21:211–217
Bhandari NN (1984) The microsporangium. In: Johri BM (ed) The embryology of angiosperms. Springer, Berlin, pp 53–157
Bhanwra RK (1988) Embryology in relation to systematic of Gramineae. Ann Bot 62(2):215–233
Carrizo Garcia C (2002) Anther wall formation in Solanaceae species. Ann Bot 90:701–706
Christensen JE, Horner HT, Lersten NR (1972) Pollen wall and tapetal orbicular wall development in Sorghum bicolour (Graminae). Am J Bot 59:43–58
Clèment C, Chavant L, Burrus M, Audran JC (1994) Anther starch variations in Lilium during pollen development. Sex Plant Reprod 7:347–356
Datta R, Chamusco KC, Chourey PS (2002) Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiol 130:1645–1656
Davis GL (1996) Systematic embryology of the angiosperms. Wiley, New York
Dorion S, Lalonde S, Saini HS (1996) Induction of male sterility in wheat by meiotic stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiol 111:137–145
Draper J, Mur LA, Jenkins G, Ghosh-Biswas GC, Bablak P, Hasterok R, Routledge AP (2001) Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiol 127:1539–1555
El-Ghazaly G, Jensen WA (1986) Studies of the development of wheat (Triticum aestivum) pollen. I Formation Pollen Wall Ubisch Bodies Grana 25:1–29
Garvin DF, Gu YQ, Hasterok R, Hazen SP, Jenkins G, Mockler TC, Mur LAJ, Vogel JP (2008) Development of genetic and genomic research resources for Brachypodium distachyon, a new model system for grass crop research. Crop Sci 48:S69–S84
Hardy CR, Stevensen DW (2000) Development of the gametophytes, flower and floral vasculature in Cochliostema odoratissumum (Commelinaceae). Bot J Linn Soc 134:131–157
Heslop-Harrison J (1964) Cell walls, cell membranes and protoplasmic connections during meiosis and pollen development. In: Linskens HF (eds) Pollen physiology and fertilization. Amsterdam, North-Holland, pp 39–47
Heslop-Harrison J, Dickinson MC (1969) Fine relationship of sporopollenin synthesis associated with tapetum and microspore in Lilium. Planta 84:199–214
Hesse M (1986) Orbicules and the ektexine are homologous sporopollenin concretions in Spermatophyta. Plant Syst Evol 153:37–48
Hong SY, Park JH, Cho SH, Yang MS, Park CM (2011) Phenological growth stages of Brachypodium distachyon: codification and description. Weed Res. doi:10.1111/j.1365-3180.2011.00877.x
Huysmans S, El-Ghazaly G, Smets E (1998) Orbicules in angiosperms: morphology, function, distribution, and relation with tapetum type. Bot Rev 64:240–272
Johri BM, Ambegaokar KB, Srivastava PS (1992) Comparative embryology of Angiosperms, vol 1. Springer, Berlin, pp 504–509
Liu CC, Huang TC (2003) Anther and pollen wall development in Dumasia miaoliensis Liu and Lu (Fabaceae). Taiwania 48:273–281
Maheshwari P (1950) An introduction to the embryology of angiosperms. McGraw-Hill, New York
Mascarenhas JP (1989) The male gametophyte of flowering plants. Plant Cell 1:657–664
Mizelle MB, Sethi R, Ashton ME, Jensen WA (1989) Development of the pollen grain and tapetum of wheat (Triticum aestivum) in untreated plants and plants treated with chemical hybridizing agent RH0007. Sex Plant Reprod 2:231–253
Opanowicz M, Vain P, Draper J, Parker D, Doonan JH (2008) Brachypodium distachyon: making hay with a wild grass. Trends Plant Sci 13:172–177
Pacini E (1990) Tapetum and microspore function. In: Blackmore S, Knox RB (eds) Microspores: evolution and ontogeny. Academic, London, pp 213–237
Pacini E (1997) Tapetum character states: analytical keys for tapetum types and activities. Can J Bot 75:1448–1459
Pacini E (2000) From anther and pollen ripening to pollen presentation. Plant Syst Evol 222:19–43
Pacini E (2010) Relationships between tapetum, loculus, and pollen during development. Int J Plant Sci 171:1–11
Pacini E, Franchi GG, Hesse M (1985) The tapetum: its form, function, and possible phylogeny in Embryophyta. Plant Syst Evol 149:155–185
Papini A, Mosti S, Brighigna L (1999) Programmed cell death events during tapetum development of angiosperms. Protoplasma 207:213–221
Polowick PL, Sawhney VK (1992) Ultrastructural changes in the cell wall, nucleus and cytoplasm of pollen mother cells during meiotic prophase I in Lycopersicum esculentum (Mill.). Protoplasma 169:139–147
Raghavan V (1988) Anther and pollen development in rice (Oryza sativa). Am J Bot 75:183–196
Risueno MC, Gimenez-Martin G, Lopez-Saez JF, Garcia MIR (1969) Origin and development of sporopollenin bodies. Protoplasma 67:361–374
Rowley JR (1963) Ubisch body development in Poa annua. Grana Palynol 4:25–36
Sato T (1968) A modified method for lead staining of thin sections. J Electr Microsc, Tokyo 17:158–159
Schrauwen JAM, Mettenmeyer T, Croes AF, Wullems GJ (1996) Tapetum-specific genes: what role do they play in male gametophyte development. Acta Bot Neerl 45:1–15
Sharma A, Singh MB, Bhalla PL (2014) Cytochemistry of pollen development in Brachypodium distachyon. Plant Syst Evol. doi:10.1007/s00606-014-0989-9
Shivanna KR (2003) Pollen biology and biotechnology. Science, New Hampshire
Solís MT, Chakrabarti N, Corredor E, Cortés-Eslava J, Rodríguez-Serrano M, Biggiogera M, Risueno MC, Testillano PS (2014) Epigenetic changes accompany developmental programmed cell death in tapetum cells. Plant Cell Physiol 55(1):16–29
Teng N, Huang Z, Xijin M, Jin B, Hu Y, Lin J (2005) Microsporogenesis and pollen development in Leymus chinensis with emphasis on dynamic changes in callose deposition. Flora 200:256–263
Vogel J, Hill T (2008) High-efficiency Agrobacterium-mediated transformation of Brachypodium distachyon inbred line Bd21-3. Plant Cell Rep 27:471–478
Wu HM, Cheung AY (2000) Programmed cell death in plant reproduction. Development 44:267–281
Zhang D, Luo X, Lu Z (2011) Cytological analysis and genetic control of rice anther development. J Genet Genomics 38:379–390
Acknowledgments
We thank Dr. Simon Crawford for technical guidance with electron microscopy and access to Advanced Microscopy Facility, The University of Melbourne. AS also thanks Dr. Martin O’Brien for helping in identifying the stages of anther and pollen development and Dr. Lim Chee Liew for helping in organizing the figures during manuscript preparation. Financial support from the Australian Research Council (ARC DPO988972) is also gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Benedikt Kost
Rights and permissions
About this article
Cite this article
Sharma, A., Singh, M.B. & Bhalla, P.L. Anther ontogeny in Brachypodium distachyon . Protoplasma 252, 439–450 (2015). https://doi.org/10.1007/s00709-014-0689-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0689-x