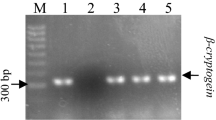
Abstract
Cotransformed hairy roots containing a gene that encodes a fungal elicitor protein, β-cryptogein, were established in Withania somnifera, a medicinal plant widely used in Indian systems of medicine. To find out whether β-cryptogein protein endogenously elicits the pathway of withasteroid biosynthesis, withaferin A and withanolide A contents along with transcript accumulation of farnesyl pyrophosphate (FPP) synthase, 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR), and sterol glycosyltransferase (SGT) were analyzed in both cryptogein-cotransformed and normal hairy roots of W. somnifera. It was observed that the withaferin A and withanolide A contents were drastically higher in normal hairy roots than cryptogein-cotransformed ones. Similar trends were also observed on the levels of transcript accumulation. Subsequently, the enzyme activity of phenylalanine ammonia lyase (PAL), one of the key enzymes of phenylpropanoid pathway, was measured in both cryptogein-cotransformed and normal hairy roots of W. somnifera along with the levels of PAL transcript accumulation. Upliftment of PAL activity was observed in cryptogein-cotransformed hairy roots as compared to the normal ones, and the PAL expression also reflected a similar trend, i.e., enhanced expression in the cryptogein-cotransformed lines. Upliftment of wall-bound ferulic acid accumulation was also observed in the cryptogein-cotransformed lines, as compared to normal hairy root lines. Thus, the outcome of the above studies suggests a metabolic shift from withanolide accumulation to phenylpropanoid biosynthesis in cryptogein-cotransformed hairy roots of W. somnifera.









Similar content being viewed by others
References
Akhtar N, Gupta P, Sangwan NS, Trivedi PK (2013) Cloning and functional characterization of 3-hydroxy-3-methylglutaryl coenzymeA reductase gene from Withania somnifera: an important medicinal plant. Protoplasma 250:613–622
Amelot N, Carrouché A, Danoun S, Bourque S, Haiech J, Pugin A, Ranjeva R, Grima-Pettenati J, Mazars C, Brière C (2011) Cryptogein, a fungal elicitor, remodels the phenylpropanoid metabolism of tobacco cell suspension cultures in a calcium-dependent manner. Plant Cell Environ 34:149–161
Amelot N, Dorlhac de Borne F, San Clemente H, Mazars C, Grima-Pettenati J, Brière C (2012) Transcriptome analysis of tobacco BY-2 cells elicited by cryptogein reveals new potential actors of calcium-dependent and calcium-independent plant defense pathways. Cell Calcium 51:117–130
Andre C, Froehlich JE, Moll MR, Benning C (2007) A heteromeric plastidic pyruvate kinase complex involved in seed oil biosynthesis in Arabidopsis. Plant Cell 19:2006–2022
Bariuari J, Canistro D, Paolini M, Ferroni F, Pedulli GF, Iori R, Valgimigli L (2005) Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in Rocket (Eruca sativa Mill.) seeds and sprouts. J Agric Food Chem 53:2475–2482
Bate NJ, Orr J, Ni W, Meromi A, Nadler-Hassan T, Doerner PW, Dixon RA, Lamb CJ, Elkind Y (1994) Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-determining step in natural product synthesis. Proc Natl Acad Sci U S A 91:7608–7612
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:1151–1154
Chakraborty SK, De BK, Bandyopadhyay T (1974) Variations in the antitumour constituents of Withania somnifera. Experientia 30:852–853
Chakraborty D, Sircar D, Mitra A (2008) Phenylalanine ammonia-lyase-mediated biosynthesis of 2-hydroxy-4-methoxybenzaldehyde in roots of Hemidesmus indicus. J Plant Physiol 165:1033–1040
Chance B, Maehly AC (1955) Assay of catalase and peroxidases. Methods Enzymol 2:764–765
Chappell J (1995) The biochemistry and molecular biology of isoprenoid metabolism. Plant Physiol 107:1–6
Chaudhuri K, Das S, Bandyopadhyay M, Zalar A, Kollmann A, Jha S, Tepfer D (2009) Transgenic mimicry of pathogen attack stimulates growth and secondary metabolite accumulation. Transgenic Res 18:121–134
Chaurasiya ND, Sangwan NS, Sabir F, Misra L, Sangwan RS (2012) Withanolide biosynthesis recruits both mevalonate and DOXP pathways of isoprenogenesis in Ashwagandha Withania somnifera L. (Dunal). Plant Cell Rep 31:1889–1897
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Dewick PM (2001) Medicinal natural product: a biosynthetic approach, 2nd edn. Wiley, New York, pp 237–241
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Donghua J, Xujun C, Kunlu W, Zejian G (2004) Expression of cryptogein in tobacco plants exhibits enhanced disease resistance and tolerance to salt stress. Chin Sci Bull 49:803–809
Frank WE, Kirson I, Lavie D, Abraham A (1980) New withanolides from a cross of a South African chemotype by chemotype II (Israel) in Withania somnifera. Phytochemistry 19:1503–1507
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Glotter E (1991) Withanolides and related ergostane-type steroids. Nat Prod Rep 8:415–440
Glotter E, Kirson I, Abraham A, Lavie D (1973) Constituents of Withania somnifera Dun. XIII-The withanolides of chemotype III. Tetrahedron 29:1353–1364
Gupta P, Akhtar N, Tewari SK, Sangwan RS, Trivedi PK (2011) Differential expression of farnesyl diphosphate synthase gene from Withania somnifera in different chemotypes and in response to elicitors. Plant Growth Regul 65:93–100
Kim OT, Kim SH, Ohyama K, Muranaka T, Choi YE, Lee HY, Kim MY, Hwang B (2010) Upregulation of phytosterol and triterpene biosynthesis in Centella asiatica hairy roots overexpressed ginseng farnesyl diphosphate synthase. Plant Cell Rep 29:403–411
Lecourieux D, Ouaked F, Pugin A, Lebrun-Garcia A (2000) Phosphoproteins involved in the signal transduction of cryptogein, an elicitor of defense reactions in tobacco. Mol Plant Microbe Interact 13:821–829
Lockley WJS, Rees HH, Goodwin TW (1976) Biosynthesis of steroidal withanolides in Withania somnifera. Phytochemistry 15:937–939
Majumdar S, Garai S, Jha S (2012) Use of the cryptogein gene to stimulate the accumulation of bacopa saponins in transgenic Bacopa monnieri plants. Plant Cell Rep 31:1899–1909
Mayer MJ, Narbad A, Parr AJ, Parker ML, Walton NJ, Mellon FA, Michael AJ (2001) Rerouting the plant phenylpropanoid pathway by expression of a novel bacterial enoyl-CoA hydratase/lyase enzyme function. Plant Cell 13:1669–1682
Mishra L, Lal P, Sangwan RS, Sangwan NS, Uniyal GC, Tuli R (2005) Unusually sulfated and oxygenated steroids from Withania somnifera. Phytochemistry 66:2702–2707
Mukherjee C, Sircar D, Chatterjee M, Das S, Mitra A (2014) Combating photooxidative stress in green hairy roots of Daucus carota cultivated under light irradiation. J Plant Physiol 171:179–187
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
O’Donohue MJ, Gousseau H, Huet JC, Tepfer D, Pernollet JC (1995) Chemical synthesis, expression and mutagenesis of a gene encoding β-cryptogein, an elicitin produced by Phytophthora cryptogea. Plant Mol Biol 27:577–586
Parr AJ, Waldron KW, Ng A, Parker ML (1996) The wall-bound phenolics of Chinese Water Chestnut (Eleocharis dulcis). J Sci Food Agric 71:501–507
Parr AJ, Ng A, Waldron KW (1997) Ester-linked phenolic components of carrot cell walls. J Agric Food Chem 45:2468–2471
Ray S, Jha S (1999) Withanolide synthesis in cultures of Withania somnifera transformed with Agrobacterium tumefaciens. Plant Sci 146:1–7
Ray S, Ghosh B, Sen S, Jha S (1996) Withanolide production by root cultures of Withania somnifera transformed with Agrobacterium rhizogenes. Planta Med 62:571–573
Ricci P, Bonnet P, Huet J-C, Sallantin M, Beauvais-Cante F, Bruneteau M, Billard V, Michel G, Pernollet J-C (1989) Structure and activity of proteins from pathogenic fungi Phytopthora eliciting necroses and acquired resistance in tobacco. Eur J Biochem 183:555–563
Rohmer M (1999) The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 16:565–574
Roja G, Heble MR, Sipahimalani AT (1991) Tissue culture of Withania somnifera: morphogenesis and withanolide synthesis. Phytother Res 5:185–187
Sharma LK, Madina BR, Chaturvedi P, Sangwan RS, Tuli R (2007) Molecular cloning and characterization of one member of 3β-hydroxysterol glucosyltransferase gene family in Withania somnifera. Arch Biochem Biophys 460:48–55
Sircar D, Mitra A (2008) Evidence for p-hydroxybenzoate formation involving phenylpropanoid chain-cleavage in hairy roots of Daucus carota. J Plant Physiol 165:407–414
Sircar D, Roychowdhury A, Mitra A (2007) Accumulation of p-hydroxybenzoic acid in hairy roots of Daucus carota. J Plant Physiol 164:1358–1366
Sokal RR, Rohlf FJ (1987) Introduction to biostatistics. WH Freeman, New York
Tepfer D, Boutteaux C, Vigon C, Aymes S, Perez V, O’Donohue MJ, Huet JC, Pernollet JC (1998) Phytophthora resistance through production of a fungal protein elicitor (ß-cryptogein) in tobacco. Mol Plant Microbe Interact 11:64–67
Vuković R, Bauer N, Ćurković-Perica M (2013) Genetic elicitation by inducible expression of ß-cryptogein stimulates secretion of phenolics from Coleus blumei hairy roots. Plant Sci 199–200:18–28
Acknowledgments
S Jha thanks David Tepfer of INRA, Versailles (France) for providing the Agrobacterium rhizogenes 9402 strain harboring a binary vector (pBIN19) containing the β-cryptogein gene. B Sil thanks the Council of Scientific and Industrial Research (CSIR), India, for the award of an individual senior research fellowship (SRF-NET). Facilities created from a research grant (SERB/SR/S0/PS/18/2011 to A Mitra and S Jha) of the Science and Engineering Research Board, Department of Science and Technology, India, for a related project, were utilized in this work.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contribution
AM, BS, and CM conceived and designed research. BS did all the experiments. BS and CM analyzed the data. SJ guided the genetic transformation experiments and took the overall responsibility. AM wrote the final manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
BS and CM contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
PCR amplification of cryptogein gene in normal hairy roots A) and crypt-cotransformed B) hairy roots of W. somnifera. (C) Positive control, A. rhizogenes strain LBA9402 harboring pBIN19-CRYPT binary vector; (M) Molecular marker (Gene Ruler 100 bp DNA ladder). (GIF 56 kb)
Supplementary Fig. 2
Amino acid sequence alignment of putative PAL gene of W. somnifera and corresponding sequences from other plant. Gene Bank accession numbers are mentioned beside each genus. (GIF 371 kb)
Rights and permissions
About this article
Cite this article
Sil, B., Mukherjee, C., Jha, S. et al. Metabolic shift from withasteroid formation to phenylpropanoid accumulation in cryptogein-cotransformed hairy roots of Withania somnifera (L.) Dunal. Protoplasma 252, 1097–1110 (2015). https://doi.org/10.1007/s00709-014-0743-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0743-8