Abstract
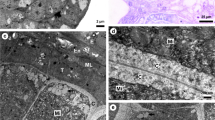
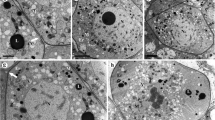
Brachypodium distachyon has emerged as a model system for forage grass and cereal grain species. Here, we report B. distachyon pollen development at the ultrastructural level. The process of microsporogenesis and microgametogenesis in B. distachyon follows the typical angiosperm pollen development sequence. Pronounced evaginations of the nuclear envelope are observed prior to meiosis, indicating active nucleocytoplasmic exchange processes. The microspore mother cells undergo meiosis and subsequent cytokinesis, forming isobilateral tetrads. Following dissolution of the callose wall and release of free and vacuolated microspores, mitotic divisions lead to the formation of mature, three-celled pollen grains. In B. distachyon, pollen wall formation begins at the tetrad stage by the formation of the exine template (primexine). The exine is tectate-columellate, comprising a foot layer and endexine. Development of the tectum and the foot layer is complete by the free microspore stage of development, with the tectum formed discontinuously. The endexine initiates in the free microspore stage but becomes compressed in mature grains. The intine layer is deposited after mitosis and comprises three layers during the mature pollen stage of development. Pore development initiates during early free microspore development stage and Brachypodium pollen has a single germination pore consisting of a slightly raised annulus surrounding a central operculum. The tapetum is of the secretory type with loss of the tapetal cell walls beginning at about the time of microsporocyte meiosis. This is the first report on ultrastructure of microsporogenesis and microgametogenesis in B. distachyon. In general, Brachypodium microsporogenesis and microgametogenesis conform to a typical grass pollen development pattern.







Similar content being viewed by others
References
Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Physiol Plant Mol Biol 62:437–460
Bevan MW, Garvin DF, Vogel JP (2010) Brachypodium distachyon genomics for sustainable food and fuel production. Curr Opin Biotech 21:211–217
Blackmore S, Wortley AH, Skvarla JJ, Rowley JR (2007) Pollen wall development in flowering plants. New Phytol 174:483–498
Christensen JE, Horner HT (1974) Pollen pore formation and its spatial orientation during microsporogenesis in the grass sorghum bicolor. Am J Bot 61:604–623
Christensen JE, Horner HT, Lersten NR (1972) Pollen wall and tapetal orbicular wall development in Sorghum bicolor (Graminae). Am J Bot 59:43–58
Draper J, Mur LA, Jenkins G, Ghosh-Biswas GC, Bablak P, Hasterok R, Routledge AP (2001) Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiol 127:1539–1555
Echlin P, Godwin H (1969) The ultrastructure and ontogeny of pollen in Helleborus foetidus L. III. The formation of the pollen grain wall. J Cell Sci 5:459–477
El-Ghazaly G, Jensen WA (1985) Studies of the development of wheat (Triticum aestivum) pollen. III. Formation of microchannels in the exine. Pollen Spores 27:5–14
El-Ghazaly G, Jensen WA (1986) Studies of the development of wheat (Triticum aestivum) pollen. I. Formation of the pollen wall and Ubisch bodies. Grana 25:1–29
El-Ghazaly G, Jensen WA (1987) Development of wheat (Triticum aestivum) pollen. II. Histochemical differentiation of wall and Ubisch bodies during development. Am J Bot 74:1396–1418
El-Ghazaly G, Jensen WA (1990) Development of wheat (Triticum aestivum) pollen wall before and after effect of a gametocide. Can J Bot 68:2509–2516
Endo M, Tsuchiya T, Hamada K, Kawamura S, Yano K, Ohshima M, Higashitani A, Watanabe M, Kawagishi-Kobayashi M (2009) High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol 50:1911–1922
Farooq M, Bramley H, Palta JA, Siddique KHM (2011) Heat stress in wheat during reproductive and grain-filling phases. Crit Rev Plant Sci 30:1–17
Fernandez MC, Rodriguez-Garcia MI (1988) Pollen wall development in Olea europaea L. New Phytol 108:91–99
Fernandez MC, Rodriguez-Garcia MI (1989) Developmental changes in the aperture during pollen grain ontogeny in Olea europaea L. New Phytol 111:717–723
Fernandez MC, Romero AT, Rodriguez-Garcia MI (1992) Aperture structure, development and function in Lycopersicon esculentum Miller (Solanaceae) pollen grains. Rev Palaebot Palynol 72:41–48
Fu JH, Lei LG, Chen LB, Qiu GZ (2001) Wall ultrastructure and cytochemistry and the longevity of pollen of three grass species. Aust J Bot 49:771–776
Garvin DF, Gu YQ, Hasterok R, Hazen SP, Jenkins G, Mockler TC, Mur LAJ, Vogel JP (2008) Development of genetic and genomic research resources for Brachypodium distachyon, a new model system for grass crop research. Crop Sci 48:S69–S84
Girin T, David LC, Chardin C, Sibout R, Krapp A, Ferrario-Mèry S, Daniel-Vedele F (2014) Brachypodium: a promising hub between model species and cereals. J Exp Bot 65:5683–5696
Harsant J, Pavlovic L, Chiu G, Sultmanis S, Sage TL (2013) High temperature stress and its effect on pollen development and morphological components of harvest index in the C3 model grass Brachypodium distachyon. J Exp Bot. doi:10.1093/jxb/ert142
Heslop-Harrison J (1963) An ultrastructural study of pollen wall ontogeny in Silene Pendula. Grana 4(1):7–24
Heslop-Harrison J (1964) Cell walls, cell membranes and protoplasmic connections during meiosis and pollen development. In: Linskens HF (eds) Pollen physiology and fertilization. Amsterdam, North-Holland, pp-39-47
Heslop-Harrison J (1966) Cytoplasmic connexions between angiosperm meiocytes. Ann Bot 30(2):221–230
Heslop-Harrison (1968a) Wall development within the microspore tetrad of Lilium longiflorum. Can J Bot 46:1185–1192
Heslop-Harrison J (1971) Wall pattern formation in angiosperm microsporogenesis. Symp Soc Exp Biol 25:277–300
Heslop-Harrison J (1979) Aspects of the structure, cytochemistry and germination of the pollen of rye (Secale cereal L.). Ann Bot 44 (Suppl. 1):1–47
Hesse M, Halbritter H, Zetter R, Weber M, Buchner R, Frosch-Radivo A, Ulrich S (2009) Pollen terminology. An illustrated handbook. Springer, New York
Jackson DD (1928) A glossary of botanic terms, 4th edn. Duckworth, London
Kreunen SS, Osborn JM (1999) Pollen and anther development in Nelumbo (Nelumbonaceae). Am J Bot 86:1662–1676
Lallemand B, Erhardt M, Heitz T, Legrand M (2013) Sporopollenin biosynthetic enzymes interact and constitute a metabolon localized to the endoplasmic reticulum of tapetum cells. Plant Physiol 162:616–625
Lin YC, Zhang ZY, He G-P (1979) Ultrastructural studies on the stamen development of rice (Oryza sativa L.). I. The ultrastructural changes in formation of the exine of pollen wall. Sunyatsenia 3:82–90 (in Chinese)
Liu L, Fan X-D (2013) Tapetum: regulation and role in sporopollenin biosynthesis in Arabidopsis. Plant Mol Bio 83:165–175
Marquez J, Seoane-Camba JA, Suarez-Cervera M (1997a) Allergenic and antigenic proteins released in the apertural sporoderm during the activation process in grass pollen grains. Sex Plant Reprod 10:269–278
Marquez J, Seoane-Camba JA, Suarez-Cervera M (1997b) The role of the intine and cytoplasm in the activation and germination processes of Poaceae pollen grains. Grana 36:328–342
Mizelle MB, Sethi R, Ashton ME, Jensen WA (1989) Development of the pollen grain and tapetum of wheat (Triticum aestivum) in untreated plants and plants treated with chemical hybridizing agent RH0007. Sex Plant Reprod 2:231–253
Opanowicz M, Vain P, Draper J, Parker D, Doonan JH (2008) Brachypodium distachyon: making hay with a wild grass. Trends Plant Sci 13:172–177
Owen HA, Makaroff CA (1995) Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. Ecotype Wassilewskija (Brassicaceae). Protoplasma 185:7–21
Prasad PVV, Boote KJ, Allen LHJR (2006) Adverse high temperature on pollen viability, seed-set, seed yield and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperature. Agr Forest Meteorol 139:237–251
Punt W, Blackmore S, Nilsson S, Thomas A LE (1994) Glossary of pollen and spore terminology. LPP Contributions series No. 1. LPP Foundation, Utrecht, Netherlands
Roland F (1971) Characterization and extraction of the polysaccharides of the intine and of the generative cell wall in the pollen grains of some Ranunculaceae. Grana 11:101–106
Romero AT, Salinas MJ, Fernandez MC (2003) Pollen wall development in Hypecoum imberbe Sm. (Fumariaceae). Grana 42:91–101
Rowley JR (1962) Stranded arrangement of sporopollenin in the exine of microspores of Poa annua. Science 137:526–528
Rowley JR (1964) Formation of the pore in pollen of Poa annua. In: Linskens HF (eds) Pollen physiology and fertilization. Amsterdam, North-Holland, pp-59-69
Rowley JR (1973) Formation of pollen exine bacules and microchannels on a glycocalyx. Grana 13:129–138
Rowley JR (1990) The fundamental structure of the pollen exine. Plant Syst Evol (Suppl) 5:13–29
Rowley JR, Dahl AO (1977) Pollen development in Artemisia vulgaris with special reference to glycocalyx materials. Pollen Spores 19:169–284
Rowley JR, Skvarla JJ (1975) The glycocalyx and initiation of exine spinules on microspores of Canna. Am J Bot 62:479–485
Sakata T, Takahashi H, Nishiyama I, Higashitani A (2000) Effects of high temperature on the development of pollen mother cells and microspores in barley Hordeum vulgare L. J Plant Res 113:395–402
Sato T (1968) A modified method for lead staining of thin sections. J. Electr. Microsc, Tokyo 17:158–159
Sharma A, Singh MB, Bhalla PL (2014b) Anther ontogeny in Brachypodium distachyon. Protoplasma. doi:10.1007/s00709-014-0689-x
Sheldon JM, Dickinson HG (1983) Determination of patterning in the pollen wall in Lilium henryi. J Cell Sci 63:191–208
Sheldon JM, Dickinson HG (1986) The relationship between the microtubular cytoskeleton and formation of the pollen wall in Lilium henryi. Planta 168:11–23
Skvarla JJ, Larson DA (1966) Fine structural studies of Zea mays pollen. I. Cell membranes and exine ontogeny. Am J Bot 53:1112–1125
Suarez-Cervera M, Guillespie L, Arcalis E, Le Thomas A, Lobreau-Callen D, Seoane-Camba JA (2001) Taxonomic significance of sporoderm structure in pollen of Euphorbiaceae: Tribes Plukenetieae and Euphorbieae. Grana 40:78–104
Takahashi M (1989) Pattern determination of the exine in Caesalpinia japonica (Leguminosae: Caesalpinioideae). Am J Bot 76:1615–1626
Takahashi M (1993) Exine initiation and substructure in pollen of Caesalpinia japonica (Leguminosae: Caesalpinioideae). Am J Bot 80:192–197
Takahashi M (1994) Exine development in Illicium religiosum Sieb. Et Zucc. (Illiciaceaec). Grana 33:309–312
Takahashi M, Kouchi J (1988) Ontogenetic development of spinous exine in Hibiscus syriacus (Malvaceae). Am J Bot 75:1549–1558
Takahashi M, Skvarla JJ (1991a) Exine pattern formation by plasma membrane in Bougainvillea spectabilis Willd. (Nyctaginaceae). Am J Bot 78:1063–1069
Takahashi M, Skvarla JJ (1991b) Development of striate exine in Ipomopsis rubula (Polemoniaceae). Am J Bot 78:1724–1731
Vinckier S, Smets E (2005) A histological study of microsporogenesis in Tarenna gracilipes (Rubiaceae). Grana 44:30–44
Wodehouse RP (1935) Pollen grains, Their structure, identification and significance in science and medicine. McGraw-Hill, New York
Zhou Q, Zhu J, Cui YL, Yang ZN (2015) Ultrastructure analysis reveals sporopollenin deposition and nexine formation at early stage of pollen wall development in Arabidopsis. Science Bulletin 60:273–276
Acknowledgments
We thank Dr. Simon Crawford for technical guidance with electron microscopy and access to Advanced Microscopy Facility, The University of Melbourne. AS also thanks Dr. Martin O’Brien for helping in identifying the stages of anther and pollen development and Dr. Lim Chee Liew for suggesting improvements in the manuscript and helping in organizing the figures during manuscript preparation. Financial support from the Australian Research Council (ARC DPO988972) is also gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Benedikt Kost
Rights and permissions
About this article
Cite this article
Sharma, A., Singh, M.B. & Bhalla, P.L. Ultrastructure of microsporogenesis and microgametogenesis in Brachypodium distachyon . Protoplasma 252, 1575–1586 (2015). https://doi.org/10.1007/s00709-015-0793-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0793-6