Abstract
Auxin and polar auxin transport have been implicated in controlling zygotic embryo development, but less is known about their role in the development of somatic embryos. The aim of this study was to determine if indole-3-acetic acid (IAA) and the PIN1 transporter participate in the induction of somatic embryogenesis (SE) and the development of somatic embryos. The results show that IAA levels gradually increase during pre-treatment and accumulate in the chloroplast. During pre-treatment and the globular stage of SE in C. canephora, auxin is distributed uniformly in all of the cells of the somatic embryo. During the subsequent stages of development, auxins are mobilized to the cells that will form the cotyledons and the root meristem. The location of the PIN transporters shifts from the plasmalemma of the protoderm cells during the globular stage to the plasmalemma of the cells that will give rise to the cotyledons and the vascular tissue in the late stages of somatic embryogenesis. The incubation of the explants in the presence of 2,3,5-triiodobenzoic acid (TIBA) produced aberrant somatic embryos, suggesting that PIN1 mediates the transport of IAA.








Similar content being viewed by others
Abbreviations
- DAPI:
-
4′,6-diamidino-2-phenylindole
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- IAA:
-
indole-3-acetic acid
- NAA:
-
1-naphthaleneacetic acid
- BA:
-
benzyl adenine
- Kin:
-
kinetin
- NPA:
-
N-1-naphthylphthalamic acid
- PAT:
-
polar auxin transport
- PBS:
-
phosphate-buffered saline
- PIN:
-
PIN-FORMED efflux carrier protein
- TIBA:
-
2,3,5-triiodobenzoic acid
References
Abrahamsson M, Valladares S, Larsson E et al (2012) Patterning during somatic embryogenesis in scots pine in relation to polar auxin transport and programmed cell death. Plant Cell Tissue Organ Cult 109:391–400. https://doi.org/10.1007/s11240-011-0103-8
Adamowski M, Friml J (2015) PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 27:20–32. https://doi.org/10.1105/tpc.114.134874
Aida M, Vernoux T, Furutani M et al (2002) Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development 129:3965–3974
Ayil-Gutiérrez BA, Galaz-Ávalos RM, Peña-Cabrera E, Loyola-Vargas VM (2013) Dynamics of the concentration of IAA and some of its conjugates during the induction of somatic embryogenesis in Coffea canephora. Plant Signal Behav 8:e26998. https://doi.org/10.4161/psb.26998
Benková E, Michniewicz M, Sauer M et al (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602. https://doi.org/10.1016/S0092-8674(03)00924-3
Choi YE, Katsumi M, Sano H (2001) Triiodobenzoic acid, an auxin polar transport inhibitor, suppresses somatic embryo formation and postembryonic shoot/root development in Eleutherococcus senticosus. Plant Sci 160:1183–1190. https://doi.org/10.1016/S0168-9452(01)00357-0
Choi YE, Kim HS, Soh WY, Yang DC (1997) Developmental and structural aspects of somatic embryos formed on medium containing 2,3,5-triiodobenzoic acid. Plant Cell Rep 16:738–744. https://doi.org/10.1007/s002990050312
Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890. https://doi.org/10.1093/nar/16.22.10881
Cueva-Agila A, Medina J, Concia L, Cella R (2016) Effects of plant growth regulator, auxin polar transport inhibitors on somatic embryogenesis and CmSERK gene expression in Cattleya maxima (Lindl.) In: Mujib A (ed) Somatic embryogenesis in ornamentals and its applications, springer, India, pp 255–267. https://doi.org/10.1007/978-81-322-2683-3_16
Denoeud F, Carretero-Paulet L, Dereeper A et al (2014) The coffee genome provides insight into the convergent evolution of caffeine biosynthesis. Science 345:1181–1184. https://doi.org/10.1126/science.1255274
Dubas E, Moravciková J, Libantová J et al (2014) The influence of heat stress on auxin distribution in transgenic B. napus microspores and microspore-derived embryos. Protoplasma 251:1077–1087. https://doi.org/10.1007/s00709-014-0616-1
Elhiti M, Stasolla C (2011) Ectopic expression of the Brassica SHOOTMERISTEMLESS attenuates the deleterious effects of the auxin transport inhibitor TIBA on somatic embryo number and morphology. Plant Sci 180:383–390. https://doi.org/10.1016/j.plantsci.2010.10.014
Folke S, Agneta E, Per G et al (1993) Compartmentation of indole-3-acetic acid metabolism in protoplasts isolated from leaves of wild-type and IAA-overproducing transgenic tobacco plants. Planta 91:274–279. https://doi.org/10.1007/BF00199760
Friml J, Vieten A, Sauer M et al (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426:147–153. https://doi.org/10.1038/nature02085
Fujimura T, Komamine A (1979) Involvement, of endogenous auxin in somatic embryogenesis in a carrot cell suspension culture. Z Pflanzenphysiol 95:13–19. https://doi.org/10.1016/S0044-328X(79)80023-9
Fujimura T (2014) Carrot somatic embryogenesis. A dream come true? Plant Biotechnol Rep 8:23–28. https://doi.org/10.1007/s11816-013-0295-y
Goran S, Einar J, Alan C (1982) Biosynthesis of indole-3-acetic acid in protoplasts, chloroplasts and a cytoplasmic fraction from barley (Hordeum vulgare L.) Planta 156:541–545. https://doi.org/10.1007/BF00392778
Göran S, Per G, Folke S, Olof O (1990) Presence of indole-3-acetic acid in chloroplasts of Nicotiana tabacum and Pinus sylvestris. Planta 180:562–568. https://doi.org/10.1007/BF02411455
Habibi N, Suthar RK, Purohit SD (2009) Role of PGRs and inhibitors in induction and control of somatic embryogenesis in Themeda quadrivalvis. Ind J Exp Biol 47:198–203
Hakman I, Hallberg H, Palovaara J (2009) The polar auxin transport inhibitor NPA impairs embryo morphology and increases the expression of an auxin efflux facilitator protein PIN during Picea abies somatic embryo development. Tree Physiol 29:483–496. https://doi.org/10.1093/treephys/tpn048
Hutchinson MJ, Murch SJ, Saxena PK (1996) Morphoregulatory role of thidiazuron: evidence of the involvement of endogenous auxin in thidiazuron-induced somatic embryogenesis of geranium (Pelargonium x hortorum bailey). J Plant Physiol 149:573–579. https://doi.org/10.1016/S0176-1617(96)80336-1
Ivanova A, Velcheva M, Denchev P et al (1994) Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiol Plant 92:85–89. https://doi.org/10.1111/j.1399-3054.1994.tb06658.x
Jen-Tsung C, Wei-Chin C (2004) TIBA affects the induction of direct somatic embryogenesis from leaf explants of Oncidium. Plant Cell Tissue Organ Cult 79:315–320. https://doi.org/10.1007/s11240-004-4613-5
Jiménez VM, Bangerth F (2001) Endogenous hormone levels in explants and in embryogenic and non-embryogenic cultures of carrot. Physiol Plant 111:389–395. https://doi.org/10.1034/j.1399-3054.2001.1110317.x
Klíma P, Lanková M, Zazimalová E (2016) Inhibitors of plant hormone transport. Protoplasma 253:1391–1404. https://doi.org/10.1007/s00709-015-0897-z
Kuhn BM, Nodzynski T, Errafi S et al (2017) Flavonol-induced changes in PIN2 polarity and auxin transport in the Arabidopsis thaliana rol1-2 mutant require phosphatase activity. Sci Rep 7:41906. https://doi.org/10.1038/srep41906
Larsson E, Sitbon F, Ljung K, von Arnold S (2008) Inhibited polar auxin transport results in aberrant embryo development in Norway spruce. New Phytol 177:356–366. https://doi.org/10.1111/j.1469-8137.2007.02289.x
Loyola-Vargas VM, De-la-Peña C, Galaz-Ávalos RM et al (2008) Plant tissue culture. In: Walker JM, Rapley R (eds) Molecular Biomethods Handbook. Humana Press, Totowa, pp 875–904. https://doi.org/10.1007/978-1-60327-375-6_50
Loyola-Vargas VM, Ochoa-Alejo N (2016) Somatic embryogenesis. An overview. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Somatic embryogenesis. Fundamental aspects and applications. Springer, Switzerland, pp 1–10. https://doi.org/10.1007/978-3-319-33705-0_1
Michalczuk L, Ribnicky DM, Cooke TJ, Cohen JD (1992) Regulation of indole-3-acetic acid biosynthetic pathways in carrot cell cultures. Plant Physiol 100:1346–1353. https://doi.org/10.1104/pp.100.3.1346
Moghaddam BE, Mesbah M, Yavari N (2000) The effect of in planta TIBA and proline treatment on somatic embryogenesis of sugar beet (Beta vulgaris L.) Euphytica 112:151–156. https://doi.org/10.1023/A:1003879914856
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nic-Can GI, Hernández-Castellano S, Kú-González A et al (2013) An efficient immunodetection method for histone modifications in plants. Plant Meth 9:47. https://doi.org/10.1186/1746-4811-9-47
Nissen P, Minocha SC (1993) Inhibition by 2,4-D of somatic embryogenesis in carrot as explored by its reversal by difluoromethylornithine. Physiol Plant 89:673–680. https://doi.org/10.1111/j.1399-3054.1993.tb05272.x
Palovaara J, Hallberg H, Stasolla C et al (2010) Expression of a gymnosperm PIN homologous gene correlates with auxin immunolocalization pattern at cotyledon formation and in demarcation of the procambium during Picea abies somatic embryo development and in seedling tissues. Tree Physiol 30:479–489. https://doi.org/10.1093/treephys/tpp126
Pescador R, Kerbauy GB, Melo Ferreira W et al (2012) A hormonal misunderstanding in Acca sellowiana embryogenesis: levels of zygotic embryogenesis do not match those of somatic embryogenesis. Plant Growth Regul 68:67–76. https://doi.org/10.1007/s10725-012-9694-2
Petrášek J, Friml J (2009) Auxin transport routes in plant development. Development 136:2675–2688. https://doi.org/10.1242/10.1242/dev.030353
Quiroz-Figueroa FR, Monforte-González M, Galaz-Ávalos RM, Loyola-Vargas VM (2006) Direct somatic embryogenesis in Coffea canephora. In: Loyola-Vargas VM, Vázquez-Flota FA (eds) Plant cell culture protocols, Humana Press, Totowa, pp 111–117. https://doi.org/10.1385/1-59259-959-1:111
Ramarosandratana AV, Van Staden J (2004) Effects of auxins and 2,3,5-triiodobenzoic acid on somatic embryo initiation from Norway spruce zygotic embryos (Picea abies). Plant Cell Tissue Organ Cult 79:105–107. https://doi.org/10.1023/B:TICU.0000049446.77837.d7
Robert HS, Friml J (2009) Auxin and other signals on the move in plants. Nat Chem Biol 5:325–332. https://doi.org/10.1038/nchembio.170
Rodríguez-Sanz H, Solís MT, López MF et al (2015) Auxin biosynthesis, accumulation, action and transport are involved in stress-induced microspore embryogenesis initiation and progression in Brassica napus. Plant Cell Physiol 56:1401–1417. https://doi.org/10.1093/pcp/pcv058
Schiavone FM, Cooke TJ (1987) Unusual patterns of somatic embryogenesis in the domesticated carrot: developmental effects of exogenous auxins and auxin transport inhibitors. Cell Differ 21:53–62. https://doi.org/10.1016/0045-6039(87)90448-9
Su YH, Zhang XS (2009) Auxin gradients trigger de novo formation of stem cells during somatic embryogenesis. Plant Signal Behav 4:574–576. https://doi.org/10.4161/psb.4.7.8730
Teale W. Palme K (2017) Naphthylphthalamic acid and the mechanism of polar auxin transport. J Exp Bot, (In press). https://doi.org/10.1093/jxb/erx323
Tétu T, Sangwan RS, Sangwan-Norreel BS (1990) Direct somatic embryogenesis and organogenesis in cultured immature zygotic embryos of Pisum sativum L. J Plant Physiol 137:102–109. https://doi.org/10.1016/S0176-1617(11)80018-0
Venkatesh K, Rani RA, Baburao N, Padmaja G (2009) Effect of auxins and auxin polar transport inhibitor (TIBA) on somatic embryogenesis in groundnut (Arachis hypogaea L.) African J Plant Sci 3:277–282
Vondráková Z, Eliásová K, Fischerová L, Vágner M (2011) The role of auxins in somatic embryogenesis of Abies alba. Cen Eur J Biol 6:587–596. https://doi.org/10.2478/s11535-011-0035-7
Weijers D, Sauer M, Meurette O et al (2005) Maintenance of embryonic auxin distribution for apical-basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell 17:2517–2526. https://doi.org/10.1105/tpc.105.034637
Xu YX, Liu Y, Chen ST et al (2014) The B subfamily of plant ATP binding cassette transporters and their roles in auxin transport. Biol Plant 58:401–410. https://doi.org/10.1007/s10535-014-0423-8
Xu Z, Zhang C, Zhang X et al (2013) Transcriptome profiling reveals auxin and cytokinin regulating somatic embryogenesis in different sister lines of cotton cultivar CCRI24. J Int Plant Biol 55:631–642. https://doi.org/10.1111/jipb.12073
Yasuda T, Fujii Y, Yamaguchi T (1985) Embryogenic callus induction from Coffea arabica leaf explants by benzyladenine. Plant Cell Physiol 26:595–597
Zambryski PC, Xu M, Stonebloom S, Burch-Smith T (2012) Embryogenesis as a model system to dissect the genetic and developmental regulation of cell-to-cell transport via plasmodesmata. In: Kragler F, Hülskamp M (eds) Short and long distance signaling. Springer, New York, pp 45–60. https://doi.org/10.1007/978-1-4419-1532-0_2
Zhu C, Wang L, Chen J et al (2017) Over-expression of KdSOC1 gene affected plantlet morphogenesis in Kalanchoe daigremontiana. Sci Rep 7:5629. https://doi.org/10.1038/s41598-017-04387-0
Žur I, Dubas E, Krzewska M et al (2015) Hormonal requirements for effective induction of microspore embryogenesis in triticale (x Triticosecale Wittm.) anther cultures. Plant Cell Rep 34:47–62. https://doi.org/10.1007/s00299-014-1686-4
Funding
This work was supported by a grant from the National Council of Science and Technology to VMLV (CONACyT; Grant No. 257436) and a scholarship from CONACyT to REML and CPH.
Author information
Authors and Affiliations
Contributions
VM conceived the idea, proposed the experiments, and drafted the manuscript. RE and AK carried out the confocal observations, CP realized the inhibition experiments, and RM realized the plant tissue culture experiments. All the authors approved the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Klaus Harter
Electronic supplementary material
Fig. S1
Control of autofluorescence. a Transmitted light differential interference images of a transverse section of leaves. b Autofluorescence in the channel of DAPI (405 nm). c Autofluorescence in the IAA channel (488 nm). d Autofluorescence in the channel of chlorophyll (650 nm). Note the very faint autofluorescence for chlorophyll in panel (d). (JPEG 29 kb)
Fig. S2
Control experiment without the IAA antibody. a, d Transmitted light differential interference images of a transverse section of globular embryos. b, c, e, f Confocal images of globular embryos. b, e Confocal images of globular embryos stained with DAPI (channel of 405 nm). c, f Control without the primary Ab against IAA and in the presence of the secondary Ab (channel of 488 nm). e, f close-up of images (b) and (c), respectively. (JPEG 140 kb)
Fig. S3
Control experiment without the PIN1 antibody. a, d Transmitted light differential interference images of a transverse section of the globular embryo. b, c, e, f Confocal images of globular embryos. b, e Confocal images of globular embryos stained with DAPI (channel of 405 nm). c, f Control without the primary Ab against PIM1 and in the presence of the secondary Ab (channel of 488 nm). e, f close-up of images (b) and (c), respectively. (JPEG 165 kb)
Fig. S4
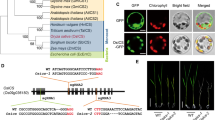
a Phylogenetic tree of C. canephora (Cc) and A. thaliana (At) PIN proteins. The tree was based on the neighbor-joining method with a bootstrap support at each node of 1000. The bar at the bottom of the figure shows the length of the branch that represents the amount of genetic change of 0.2. The protein sequences from A. thaliana were retrieved from Phytozome v7.0 database. C. canephora PIN sequences were obtained from the website providing the genome of C. canephora (http://coffee-genome.org/) using the Ugene platform (http://ugene.net/). b Amino acid sequence alignment of the transmembrane region of A. thaliana PIN1 (AtPIN1) and C. canephora PIN1 (CcPIN1 and CcPINb). The alignment was carried out using the algorithm published by Corpet (1988) (JPEG 967 kb)
Rights and permissions
About this article
Cite this article
Márquez-López, R.E., Pérez-Hernández, C., Ku-González, Á. et al. Localization and transport of indole-3-acetic acid during somatic embryogenesis in Coffea canephora . Protoplasma 255, 695–708 (2018). https://doi.org/10.1007/s00709-017-1181-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1181-1