Abstract
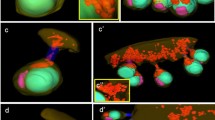
To analyze the release of mitochondrial material, a process that is believed to be (i) induced by the VASA protein derived from germplasm granules, and (ii) which appears to play an important role during meiotic differentiation, the localization of the CYTB protein was studied in the process of spermatogenesis of the bivalve mollusk Ruditapes philippinarum (Manila clam). It was found that in early spermatogenic cells, such as spermatogonia and spermatocytes, the CYTB protein shows dispersion in the cytoplasm following the total disaggregation of VASA-invaded mitochondria, what is called here as “destructive mitochondrial effusion (DME).” It was found that the mitochondria of the maturing sperm cells also uptake VASA. It is accompanied by extramitochondrial transmembrane localization of CYTB assuming mitochondrial content release without mitochondrion demolishing. This phenomenon is called here as “nondestructive mitochondrial effusion (NDME).” Thus, in the spermatogenesis of the Manila clam, two patterns of mitochondrial release, DME and NDME, were found, which function, respectively, in early spermatogenic cells and in maturing spermatozoa. Despite the morphological difference, it is assumed that both DME and NDME have a similar functional nature. In both cases, the intramitochondrial localization of VASA coincides with the extramitochondrial localization of the mitochondrial matrix.





Similar content being viewed by others
References
Ables E (2015) Drosophila oocytes as a model for understanding meiosis: an educational primer to accompany “Corolla is a novel protein that contributes to the architecture of the synaptonemal complex of Drosophila.” Genetics 199:17–23
Amikura R, Kashikawa M, Nakamura A, Kobayashi S (2001) Presence of mitochondria-type ribosomes outside mitochondria in germplasm of Drosophila embryos. PNAS 98:9133–9138
Amikura R, Sato K, Kobayashi S (2005) Role of mitochondrial ribosome dependent translation in germline formation in Drosophila embryos. Mech Dev 122:1087–1093
Aratani S, Fujii R, Oishi T, Fujita H, Amano T, Ohshima T, Hagiwara M, Fukamizy A, Nakajima T (2001) Dual roles of RNA helicase A in CREB-dependent transcription. Mol Cell Biol 2:4460–4469
Bergstrom CL, Beales PA, Lv Y, Vanderlick TK, Groves JT (2013) Cytochrome c causes pore formation in cardiolipin-containing membranes. PNAS 110:6269–6274
Carré D, Djediat C, Sardet C (2002) Formation of a large Vasa-positive germ granule and its inheritance by germ cells in the enigmatic chaetognaths. Development 129:661–670
Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP (2000) The human VASA gene is specifically expressed in the germ cell lineage. PNAS 97:9585–9590
Cordero D, Delgado M, Liu B, Ruesink J, Saavedra C (2017) Population genetics of the Manila clam (Ruditapes philippinarum) introduced in North America and Europe. Nature: Scientific Reports 7, Article number: 39745. 3
Delgado M, Perez-Camacho A (2007) Comparative study of gonadal development of Ruditapes philippinarum (Adams and Reeve) and Ruditapes decussatus (L.) (Mollusca: Bivalvia): influence of temperature. Scientia Marina 71:471–484
Fernández Robledo JA, Yadavalli R, Allam B, Pales-Espinosa E, Gerdol M, Greco S, Stevick RJ, Gómez-Chiarri M, Zhang Y, Heil CA, Tracy AN, Bishop-Bailey D, Metzger MJ (2019) From the raw bar to the bench: bivalves as models for human health. Dev Comp Immunol 92:260. https://doi.org/10.1016/j.dci.2018.11.020
Findley SD, Tamanaha M, Clegg NJ, Ruohola-Baker H (2003) Maelstrom, a Drosophila spindle-class gene, encodes a protein that colocalizes with Vasa and RDE1/AGO1 homolog, aubergine, in nuage. Development 130:859–871
Ghiselli F, Maurizii MG, Reunov A, Ariño-Bassols H, Cifaldi C, Pecci A, Alexandrova Y, Bettini S, Passamonti M, Franceschini V, Milani L (2019) Natural heteroplasmy and mitochondrial inheritance in bivalve molluscs. Integr Comp Biol 59:1016–1032
Gur Y, Breitbart H (2006) Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev 20:411–416
Gur Y, Breitbart H (2008) Protein synthesis in sperm: dialog between mitochondria and cytoplasm. Mol Cell Endocrinol 282:45–55
Gustafson EA, Wessel GM (2010) DEAD-box helicases: posttranslational regulation and function. Biochem Biophys Res Commun 395:1–6
Hay B, Jan LY, Jan YN (1990) Localization of vasa, a component of Drosophila polar granules, in maternal effect mutants that alter embryonic anteroposterior polarity. Development 109:425–433
Jangamreddy JR, Los MJ (2012) Mitoptosis, a novel mitochondrial death mechanism leading predominantly to activation of autophagy. Hepat Mon 12:e6159. https://doi.org/10.5812/hepatmon.6159
Jankowsky E (2011) RNA helicases at work: binding and rearranging. Trends Biochem Sci 36:19–29
Kawasaki T, Siegfried KR, Sakai N (2016) Differentiation of zebrafish spermatogonial stem cells to functional sperm in culture. Development 143:566–574
Kobayashi S, Amikura R, Mukai M (1998) Localization of mitochondrial large ribosomal RNA in germplasm of Xenopus embryos. Curr Biol 8:1117–1120
Kobayashi S, Amikura R, Okada M (1993) Presence of mitochondrial large ribosomal RNA outside mitochondria in germplasm of Drosophila melanogaster. Science 260:5113–1521
Krawetz SA, De Rooij DG, Hedger MP (2009) Molecular aspects of male fertility. international workshop on molecular andrology. EMBO Rep 10:1087–1092
Lasko PF, Ashburner M (1988) The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature 335:611–617
Lyamzaev KG, Nepryakhina OK, Saprunova VB, Bakeeva LE, Pletjushkina OY, Chernyak BV, Skulachev VP (2008) Novel mechanism of elimination of malfunctioning mitochondria (mitoptosis): formation of mitoptotic bodies and extrusion of mitochondrial material from the cell. Biochim Biophys Acta 1777:817–825. https://doi.org/10.1016/j.bbabio.2008.03.027
Milani L, Ghiselli F, Maurizii MG, Passamonti M (2011) Doubly uniparental inheritance of mitochondria as a model system for studying germ line formation. PLoS ONE 6(11):e28194. https://doi.org/10.1371/journal.pone.0028194
Milani L, Ghiselli F, Pecci A, Maurizii MG, Passamonti M (2015) The expression of a novel mitochondrially encoded gene in gonadic precursors may drive paternal inheritance of mitochondria. PLoS ONE 10(9):e0137468. https://doi.org/10.1371/journal.pone.0137468
Milani L, Pecci A, Ghiselli F, Passamonti M, Lazzari M, Franceschini V, Maurizii MG (2018) Germ cell line during the seasonal sexual rest of clams: finding niches of cells for gonad renewal. Histochem Cell Biol 148:157–171. https://doi.org/10.1007/s00418-017-1607-z
Mochizuki K, Nishiyama-Fujisawa C, Fujisawa T (2001) Universal occurrence of the vasa-related genes among metazoans and their germline expression in Hydra. Dev Genes 211:299–308
Nakamura N (2013) Ubiquitination regulates the morphogenesis and function of sperm organelles. Cells 2:732–750. https://doi.org/10.3390/cells2040732
Pickles S, Vigié P, Youle RJ (2018) Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol 28:170–185. https://doi.org/10.1016/j.cub.2018.01.004
Raz E (2000) The function and regulation of vasa-like genes in germ-cell development. Genome Biol 1(1017):1–6
Reunov AA (2006) Structures related to the germplasm in mouse. Zygote 14:231–238. https://doi.org/10.1017/S0967199406003789
Reunov AA (2013) Premeiotic transformation of germplasm-related structures during the sea urchin spermatogenesis. Zygote 21:95–101. https://doi.org/10.1017/S0967199411000402
Reunov AA, Alexandrova YN, Reunova YA, Komkova AV, Milani L (2019) Germplasm provides clues on meiosis: the concerted action of germplasm granules and mitochondria in gametogenesis of the clam Ruditapes philippinarum. Zygote 27:25–35. https://doi.org/10.1017/S0967199418000588
Reunov AA, Au DW, Alexandrova YN, Chiang MW, Wan MT, Yakovlev KV, Reunova YA, Komkova AV, Cheung NK, Peterson DR, Adrianov AV (2020) Germplasm-related structures in marine medaka gametogenesis; novel sites of Vasa localization and the unique mechanism of germplasm granule arising. Zygote 28:9–23. https://doi.org/10.1017/S0967199419000546
Reunov AA, Reunova YA (2016) Pre-meiotic transformation of germplasm-related structures during male gamete differentiation in Xenopus laevis. Zygote 24:42–47. https://doi.org/10.1017/S0967199414000690
Reunov AA, Yakovlev K, Hu J, Reunova YA, Komkova AV, Alexandrova YN, Pimenova EA, Tiefenbach J, Krause H (2019) Close association between vasa-positive germplasm granules and mitochondria correlates with cytoplasmic localization of 12S and 16S mtrRNAs during zebrafish spermatogenesis. Differentiation 109:34–41
Reunov AA, Yurchenko OV, Alexandrova YN, Radashevsky VI (2009) Spermatogenesis in Boccardiella hamata (Polychaeta: Spionidae) from the sea of Japan: sperm formation mechanisms as characteristics for future taxonomic revision. Acta Zoologica 91:447–456
Sakurai I, Horii T, Murakami O, Nakao S (1998) Population dynamics and stock size prediction for the sunray surf clam Mactra chinensis at Tomakomai, southwest Hokkaido, Japan. Fish Bull 96:344–351
Sanjo H, Komeya M, Sato T, Abe T, Katagiri K, Yamanaka H, Ino Y, Arakawa N, Hirano H, Yao T, Asayama Y, Matsuhisa A, Yao M, Ogawa T (2018) In vitro mouse spermatogenesis with an organ culture method in chemically defined medium. PLoS ONE 13(2):e0192884
Silva AF, Escada-Rebelo S, Amaral S, Tavares RS, Schlatt S, Ramalho-Santos J, Mota PC (2018) Can we induce spermatogenesis in the domestic cat using an in vitro tissue culture approach? PLoS ONE 13(2):e0191912
Skulachev VP (2000) Mitochondria in the programmed death phenomena; a principle of biology: “it is better to die than to be wrong.” IUBMB Life 49:365–373
Skulachev VP (2006) Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis 11:473–485
Sunagara T, Saito Y, Kawamura K (2006) Postembryonic epigenesist of Vasa-positive germ cells from aggregated hemoblasts in the colonial ascidian Botryllus primigenus. Develop Growth Differ 48:87–100
Toyooka Y, Tsunekawa N, Takahashi Y, Matsui Y, Satoh M, Noce T (2000) Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev 93:139–149
Van der Schatte Olivier A, Jones L, Le Vay L, Christie M, Wilson J, Malham SK (2020) A global review of the ecosystem services provided by bivalve aquaculture. Rev Aquac 12:3–25
Villegas J, Araya P, Bustos-Obregon E, Burzio LO (2002) Localization of the 16S mitochondrial rRNA in the nucleus of mammalian spermatogenic cells. Mol Hum Reprod 8:977–983
Acknowledgments
This study was funded by the Italian Ministry of Education, University and Research MIUR-SIR Program (Grant Number RBSI14G0P5) funded to LM. We are deeply indebted to Mr. D.F. Fomin (National Scientific Centre of Marine Biology, Far Eastern Branch of Russian Academy of Sciences, Vladivostok, Russia) for the helpful assistance during our observations using the electron microscopes. Some part of the analyses by electron microscopy were performed in the Microscopy Facility at St. Francis Xavier University (Antigonish, NS, Canada).
Author information
Authors and Affiliations
Contributions
AR designed the study, implemented most of the TEM work, and was a major contributor to writing the manuscript. YA performed material collection/processing and part of the TEM analysis. AK performed part of the TEM analysis. YR performed part of the TEM analysis and the quantitative analysis. EP performed part of the TEM analysis. EV performed the SEM analysis. LM designed the antibody generation, performed material collection/processing, provided images, and was a contributor to discussion and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare we have no competing interests.
Additional information
Handling Editor: Georg Krohne.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reunov, A., Alexandrova, Y., Komkova, A. et al. VASA-induced cytoplasmic localization of CYTB-positive mitochondrial substance occurs by destructive and nondestructive mitochondrial effusion, respectively, in early and late spermatogenic cells of the Manila clam. Protoplasma 258, 817–825 (2021). https://doi.org/10.1007/s00709-020-01601-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01601-1