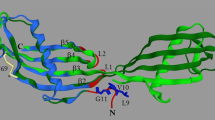
Abstract
CMS1MS2 (CC-Ib) from Carica candamarcensis (Vasconcellea cundinamarcensis) is a cysteine proteinase found as a single polypeptide containing 213 residues of 22,991 Da. The enzyme was purified by three chromatographic steps, two of them involving cationic exchange. Crystals of CMS1MS2 complexed with E-64 were obtained by the hanging drop vapor-diffusion method at 291 K using ammonium sulfate and polyethylene glycol 4000/8000 as precipitant. The complex CMS1MS2-E-64 crystallized in the tetragonal space group P41212 with unit-cell parameters; a = b = 73.64, c = 118.79 Å. The structure was determined by Molecular Replacement and refined at 1.87 Å resolution to a final R factor of 16.2 % (R free = 19.3 %). Based on the model, the structure of CMS1MS2 (PDB 3IOQ) ranks as one of the least basic cysteine isoforms from C. candamarcensis, is structurally closer to papain, caricain, chymopapain and mexicain than to the other cysteine proteinases, while its activity is twice the activity of papain towards BAPNA substrate. Two differences, one in the S2 subsite and another in the S3 subsite of CMS1MS2 may contribute to the enhanced activity relative to papain. In addition, the model provides a structural basis for the sensitivity of CMS1MS2 to inhibition by cystatin, not shown by other enzymes of the group, e.g., glycyl endopeptidase and CMS2MS2.



Similar content being viewed by others
References
Arroyo-Reyna A, Hernandez-Arana A, Arreguin-Espinosa R (1994) Circular dichroism of stem bromelain: a third spectral class within the family of cysteine proteinases. Biochem J 300:107–110
Ayello E, Cuddigan JE (2004) Debridement: controlling the necrotic/cellular burden. Adv Skin Wound Care 17:66–75
Azarkan M, Dibiani R, Baulard C, Baeyens-Volant D (2006) Effects of mechanical wounding on Carica papaya cysteine endopeptidases accumulation and activity. Int J Biol Macromol 38:216–224
Báez R, Lopes MT, Salas CE, Hernández M (2007) In vivo antitumoral activity of stem pineapple (Ananas comosus) bromelain. Planta Med 73:1377–1383
Baeza G, Correa D, Salas CE (1990) Proteolytic enzymes in C. candamarcensis. J Sci Food Agric 51:1–9
Baker EL, Baker WL, Cloney DJ (2007) Resolution of a phytobezoar with Aldoph’s Meat Tenderizer. Pharmacother 27:299–302
Barrett AJ, Buttle DJ (1985) Names and numbers of papaya proteinases. Biochem J 228:527
Batkin S, Taussig S, Szekerczes J (1988) Modulation of pulmonary metastasis (Lewis lung carcinoma) by bromelain, an extract of the pineapple stem (Ananas comosus). Cancer Invest 6:241–242
Bode W, Engh R, Musil D, Thiele U, Huber R, Karshikov A, Brzin J, Kos J, Turk V (1988) The 2.0 A X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J 7:2593–2599
Bravo LM, Hermosilla L, Salas CE (1994) A biochemical comparison between latex from C. candamarcensis and C. papaya. Braz J Med Biol Res 26:2831–2842
Brien S, Lewith G, Walker A, Hicks S, Middleton D (2004) Bromelain as a Treatment for Osteoarthritis: a Review of Clinical Studies. Evid Based Complement Alternat Med 1:251–257
Cabral H, Leopoldino AM, Tajara EH, Greene LJ, Faça VM, Mateus RP, Ceron CR, de Souza Judice WA, Juliano L, Bonilla-Rodriguez GO (2006) Preliminary functional characterization, cloning and primary sequence of Fastuosain, a cysteine peptidase isolated from fruits of Bromelia fastuosa. Protein Pept Lett 13:83–89
CCP4 Program Suite 6.0.2 (1994) Collaborative computational project number 4. Acta Cryst D50:760–763
Chobotova K, Vernallis AB, Majid FA (2010) Bromelain’s activity and potential as an anti-cancer agent: Current evidence and perspectives. Cancer Lett 290:148–156
Corrêa NC, Mendes IC, Gomes MT, Kalapothakis E, Chagas BC, Lopes MT, Salas CE (2011) Molecular cloning of a mitogenic proteinase from Carica candamarcensis: its potential use in wound healing. Phytochemistry 72:1947–1954
DeLano WL (2002) The PyMOL molecular graphics system. DeLano Scientific, Palo Alto, CA, USA. http://www.pymol.org
Drenth J, Jansonius JN, Koekoek R, Swen HM, Wolthers BG (1968) Structure of papain. Nature (London) 218:929–932
Dubois T, Kleinschmidt T, Schnek AG, Looze Y, Braunitzer G (1988) The thiol proteinases from the latex of Carica papaya L.: the primary structure of proteinase omega. Biol Chem Hoppe-Seyler 369:741–754
Ford CN, Reinhard ER, Yeh D, Syrek D, De Las Morenas A, Bergman SB, Williams S, Hamori CA (2002) Interim analysis of a prospective, randomized trial of vacuum-assisted closure versus the healthpoint system in the management of pressure ulcers. Ann Plast Surg 49:55–61
Furie B, Furie BC (1988) The molecular base of blood coagulation. Cell 53:505–518
Gaspani L, Limiroli E, Ferrario P, Bianchi M (2002) In vivo and in vitro effects of bromelain on PGE(2) and SP concentrations in the inflammatory exudate in rats. Pharmacol 65:83–86
Gass J, Bethune MT, Siegel M, Spencer A, Khosla C (2007) Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterol 133:472–480
Gavira JA, Gonzalez-Ramirez LA, Oliver-Salvador MC, Soriano-Garcia M, Garcia-Ruiz JM (2007) Structure of the mexicain-E-64 complex and comparison with other cysteine proteases of the papain family. Acta Cryst D63:555–563
Gomes MTR, Mello VJ, Rodrigues KC, Bemquerer MP, Lopes MTP, Faça VM, Salas CE (2005) Isolation of two plant proteinases in latex from Carica candamarcensis acting as mitogens for mammalian cells. Planta Med 71:244–248
Gomes MTR, Bemquerer MP, Lopes MTP, Richardson M, Oyama S, Salas CE (2007) The structure of CMS2MS2, a mitogenic protein isolated from latex of Carica candamarcensis. Biol Chem 388:819–822
Gomes MT, Teixeira RD, Ribeiro H, Turchetti AP, Junqueira CF, Lopes MT, Salas CE, Nagem RA (2008) Purification, crystallization and preliminary X-ray analysis of CMS1MS2: a cysteine proteinase from Carica candamarcensis latex. Acta Cryst F64:492–494
Gomes FS, Spínola CV, Ribeiro HA, Lopes MT, Cassali GD, Salas CE (2010a) Wound-healing activity of a proteolytic fraction from Carica candamarcensis on experimentally induced burn. Burns 36:277–283
Gomes MT, Ribeiro HA, Lopes MT, Guzman F, Salas CE (2010b) Biochemical comparison of two proteolytic enzymes from Carica candamarcensis: structural motifs underlying resistance to cystatin inhibition. Phytochemistry 71:524–530
Grabowska E, Eckert K, Fichtner I, Schulze-Forster K, Maurer H (1997) Bromelain proteases suppress growth invasion and lung metastasis of B16F10 mouse melanoma cells. Int J Oncol 11:243–248
Gravina de Moraes M, Termignoni C, Salas CE (1994) Biochemical characterization of a new cysteine endopeptidase from Carica candamarcensis L. Plant Sci 102:11–18
Grzonka Z, Jankowska E, Kasprzykowski F, Kasprzykowska R, Lankiewicz L, Wiczk W, Wieczerzak E, Ciarkowski J, Drabik P, Janowski R, Kozak M, Jaskólski M, Grubb (2001) Structural studies of cysteine proteases and their inhibitors. Acta Biochim Pol 48:1–20
Heinig M, Frishman D (2004) STRIDE: a web server for secondary structure assignment from known atomic coordinates of proteins. Nucl Acids Res 32:W500–W502
Janowski R, Kozak M, Jankowska E, Grzonka Z, Jaskólski M (2004) Two polymorphs of a covalent complex between papain and a diazomethylketone inhibitor. J Pept Res 64:141–150
Kyndt T, Van Damme EJ, Van Beeumen J, Gheysen G (2007) Purification and characterization of the cysteine proteinases in the latex of Vasconcellea spp. FEBS J 274:451–462
Maes D, Bouckaert J, Poortmans F, Wyns L, Looze Y (1996) Structure of chymopapain at 1.7 A resolution. Biochemistry 35:16292–16298
Melano E, Rodriguez HL, Carrillo R Jr, Dillon L (2004) The effects of Panafil when using topical negative pressure to heal an infected sternal wound. J Wound Care 13:425–426
Mello VJ, Gomes MTR, Rodrigues KCL, Sanchez EF, Lopes MTP, Salas CE (2006) In: Govil JN, Singh VK, Arunachalam C (eds) Recent progress in medicinal plants Drug development from molecules, vol 11. Studium Press, LLC, Houston, USA, pp 211–224
Mello VJ, Gomes MTR, Lemos FO, Delfino JL, Andrade SP, Lopes MTP, Salas CE (2008) The gastric ulcer protective and healing role of cysteine proteinases from Carica candamarcensis. Phytomed 15:237–244
Mitchel REJ, Chaiken IM, Smith EL (1970) The complete amino acid sequence of papain. J Biol Chem 245:3485–3492
Morris AL, MacArthur MW, Hutchinson EG, Thornton JM (1992) Stereochemical quality of protein structure coordinates. Proteins 12:345–364
Moutim V, Silva LG, Lopes MTP, Wilson-Fernandes G, Salas CE (1999) Spontaneous processing of peptides during coagulation of latex from Carica papaya. Plant Sci 142:115–121
Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Cryst D53:240–255
Mynott TL, Ladhams A, Scarmato P, Engwerda CR (1999) Bromelain, from pineaple stems, proteolytically blocks activation of extracellular regulated kinase-2 in T cells. J Immunol 163:2568–2575
Navaza J (1994) AMoRe: an automated package for molecular replacement. Acta Cryst A50:157–163
O’Hara BP, Hemmings AM, Buttle DJ, Pearl LH (1995) Crystal structure of glycyl endopeptidase from Carica papaya: a cysteine endopeptidase of unusual substrate specificity. Biochemistry 34:13190–13195
Otwinowski Z, Minor W (1997) Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol 276:307–326
Pereira MT, Lopes MT, Meira O, Salas CE (2001) Purification of a cysteine proteinase from Carica candamarcensis L and cloning of a genomic putative fragment coding for this enzyme. Protein Expr Purif 22:249–257
Pickersgill RW, Sumner IG, Goodenough PW (1990) Preliminary crystallographic data for protease omega. Eur J Biochem 190:443–444
Rawlings ND, Barrett AJ (1993) Evolutionary families of peptidases. Biochem J 290:205–218
Ritonja A, Buttle DJ, Rawlings ND, Turk V, Barrett AJ (1989) Papaya proteinase IV aminoacid sequence. FEBS Lett 258:109–112
Schwede T, Kopp J, Juex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Silva LG, Garcia O, Lopes MTP, Salas CE (1997) Changes in protein profile during coagulation of latex from Carica papaya. Braz J Med Biol Res 30:615–619
Silva CA, Gomes MTR, Ferreira RS, Rodrigues KCL, do Val CG, Lopes MTP, Mello VJ, Salas CE (2003) A mitogenic protein fraction in latex from Carica candamarcensis. Planta Med 69:926–932
Solís-Mendiola S, Arroyo-Reyna A, Hernández-Arana A (1992) Circular dichroism of cysteine proteinases from papaya latex. Evidence of differences in the folding of their polypeptide chains. Biochim Biophys Acta 1118:288–292
Soplin SP, Millones EA, Campos JLA, Deza LG, Rios EJ, Sanchez IB, Benítez MR, Pando LG, Panizo RS, Merino C del C, Rivera SJC (1996) Informe Nacional para la Conferencia Técnica Internacional de FAO sobre los Recursos Fitogenéticos
Sreerama N, Woody RW (1993) A self-consistent method for the analysis of protein secondary structure from circular dichroism. Anal Biochem 209:32–44
Stepek G, Lowe AE, Buttle DJ, Duce IR, Behnke JM (2006) In vitro and in vivo anthelmintic efficacy of plant cysteine proteinases against the rodent gastrointestinal nematode. Parasitol 132:681–689
Teixeira RD, Ribeiro HA, Gomes MT, Lopes MT, Salas CE (2008) The proteolytic activities in latex from Carica candamarcensis. Plant Physiol Biochem 46:956–961
Varughese KI, Su Y, Cromwell D, Hasnain S, Xuong NH (1992) Crystal structure of an actinidin-E-64 complex. Biochemistry 31:5172–5176
Wald M, Závadová E, Poucková P, Zadinová M, Boubelik M (1998) Polyenzyme preparation Wobe-Mugos inhibits growth of solid tumors and development of experimental metastases in mice. Life Sci 62:43–48
Wald M, Olejár T, Sebková V, Zadinová M, Boubelík M, Poucková P (2001) Mixture of trypsin, chymotrypsin and papain reduces formation of metastases and extends survival time of C57Bl6 mice with syngeneic melanoma B16. Cancer Chemother Pharmacol 47:S16–S22
Walraevens V, Vandermeers-Piret MC, Vandermeers A, Gourlet P, Robberecht P (1999) Isolation and primary structure of the CCI papain-like cysteine proteinases from the latex of Carica candamarcensis hook. Biol Chem 380:485–488
Walreavens V, Jaziri M, Van Beeumen J, Schneck AG, Kleinschmidt T, Looze Y (1993) Isolation and preliminary characterization of the cysteine-proteinases from the latex of Carica candamarcensis Hook. Biol Chem Hoppe-Seyler 374:501–506
Watson C, Yaguchi M, Lynn KR (1990) The aminoacid sequence of chymopapain from Carica papaya. Biochem J 266:75–81
Zhao B, Janson CA, Amegadzie BY, D’Alessio K, Griffin C, Hanning CR, Jones C, Kurdyla J, McQueney M, Qiu X, Smith WW, Abdel-Meguid SS (1997) Crystal structure of human osteoclast cathepsin K complex with E-64. Nat Struct Biol 4:109–111
Acknowledgments
The research was funded by CNPq, CAPES and Fapemig. We thank Dr. Abraham Schnaiderman for his financial aid to this research and the Brazilian Synchrotron Light Laboratory (LNLS) for providing access to their facilities for X-ray crystallography experiments. Each author confirms that there is no conflict of interest in connection with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomes, M.T.R., Teixeira, R.D., Lopes, M.T.P. et al. X-ray crystal structure of CMS1MS2: a high proteolytic activity cysteine proteinase from Carica candamarcensis . Amino Acids 43, 2381–2391 (2012). https://doi.org/10.1007/s00726-012-1318-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1318-7