Abstract
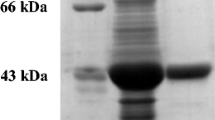
Rhizobium sp. strain TAL1145 catabolizes mimosine, which is a toxic non-protein amino acid present in Leucaena leucocephala (leucaena). The objective of this investigation was to study the biochemical and catalytic properties of the enzyme encoded by midD, one of the TAL1145 genes involved in mimosine degradation. The midD-encoded enzyme, MidD, was expressed in Escherichia coli, purified and used for biochemical and catalytic studies using mimosine as the substrate. The reaction products in the enzyme assay were analyzed by HPLC and mass spectrometry. MidD has a molecular mass of ~45 kDa and its catalytic activity was found to be optimal at 37 °C and pH 8.5. The major product formed in the reaction had the same retention time as that of synthetic 3-hydroxy-4-pyridone (3H4P). It was confirmed to be 3H4P by MS/MS analysis of the HPLC-purified product. The K m, V max and K cat of MidD were 1.27 × 10−4 mol, 4.96 × 10−5 mol s−1 mg−1, and 2,256.05 s−1, respectively. Although MidD has sequence similarities with aminotransferases, it is not an aminotransferase because it does not require a keto acid as the co-substrate in the degradation reaction. It is a pyridoxal-5′-phosphate (PLP)-dependent enzyme and the addition of 50 μM hydroxylamine completely inhibited the reaction. However, the supplementation of the reaction with 0.1 μM PLP restored the catalytic activity of MidD in the reaction containing 50 μM hydroxylamine. The catalytic activity of MidD was found to be specific to mimosine, and the presence of its structural analogs including l-tyrosine, l-tryptophan and l-phenylalanine did not show any competitive inhibition. In addition to 3H4P, we also identified pyruvate and ammonia as other degradation products in equimolar quantities of the substrate used. The degradation of mimosine into a ring compound, 3H4P with the release of ammonia indicates that MidD of Rhizobium sp. strain TAL1145 is a C–N lyase.








Similar content being viewed by others
Abbreviations
- HPLC:
-
High-performance liquid chromatography
- PLP:
-
Pyridoxal-5′-phosphate
- 3H4P:
-
3-Hydroxy-4-pyridone
- 3,4-DHP:
-
3,4-Dihydroxypyridine
- 2,3-DHP:
-
2,3-Dihydroxypyridine
- α-KG:
-
α-Ketoglutarate
- aq:
-
Aqueous
References
Alfano JR, Kahn ML (1993) Isolation and characterization of a gene coding for a novel aspartate aminotransferase from Rhizobium meliloti. J Bacteriol 175(13):4186–4196
Borthakur D, Soedarjo M (1999) Isolation and characterization of a DNA fragment containing genes for mimosine degradation from Rhizobium sp. strain TAL1145. Highlights of Nitrogen Fixation Research, New York, Kluwer/Plenum
Borthakur D, Soedarjo M et al (2003) The mid genes of Rhizobium sp. strain TAL1145 are required for degradation of mimosine into 3-hydroxy-4-pyridone and are inducible by mimosine. Microbiology 149(2):537–546
Crounse RG, Maxwell JD et al (1962) Inhibition of growth of hair by mimosine. Nature 194:694–695
Dewreede S, Wayman O (1970) Effect of mimosine on the rat fetus. Teratology 3(1):21–27
El-Sayed ASA (2011) Purification and characterization of a new l-methioninase f rom solid cultures of Aspergillus flavipes. J Microbiol 49(1):130–140
Fowden L (1964) The chemistry and metabolism of recently isolated amino acids. Annu Rev Biochem 33(1):173–204
Fox PM, Borthakur D (2001) Selection of several classes of mimosine-degradation-defective Tn3Hogus-insertion mutants of Rhizobium sp. strain TAL1145 on the basis of mimosine-inducible GUS activity. Can J Microbiol 47(6):488–494
Garcia GW, Ferguson TU et al (1996) The nutritive value and forage productivity of Leucaena leucocephala. Anim Feed Sci Technol 60(1–2):29–41
Gout E, Bligny R et al (1992) Regulation of intracellular pH values in higher plant cells. Carbon-13 and phosphorus-31 nuclear magnetic resonance studies. J Biol Chem 267(20):13903–13909
Hamilton RI, Donaldson LE et al (1968) Enlarged thyroid glands in calves born to heifers fed a sole diet of Leucaena leucocephala. Aust Vet J 44(10):484
Hegarty MP, Lee CP et al (1979) The goitrogen 3-Hydroxy-4(1H)-Pyridone, a RuminaI metabolite from Leucaena Leucocephala: effects in mice and rats. Aust J Biol Sci 32(1):27–40
Joshi HS (1968) The effect of feeding on Leucaena leucocephala (Lam) de Wit. on reproduction in rats. Aust J Agric Res 19(2):341–352
Krupka HI, Huber R et al (2000) Crystal structure of cystalysin from Treponema denticola: a pyridoxal 5′-phosphate-dependent protein acting as a haemolytic enzyme. EMBO J 19(13):3168–3178
Lin JY, Shih YM et al (1962) Studies on the mechanism of toxicity of mimosine (β-(N-[3-hydroxypyridone])-α-aminopropionic acid). (1) Studies of the reactions of mimosine and pyridoxal 5-phosphate using the spectrophotometric method. J Formos Med Assoc 61:997–1003
McKay IA, Dilworth MJ et al (1988) C4-dicarboxylate metabolism in free-living and bacteroid forms of Rhizobium leguminosarum MNF3841. J Gen Microbiol 134(6):1433–1440
Mehta PK, Hale TI et al (1993) Aminotransferases: demonstration of homology and division into evolutionary subgroups. Eur J Biochem 214(2):549–561
Smith IK, Fowden L (1966) A study of mimsine toxicity in plants. J Exp Bot 17(53):750–761
Soedarjo M, Borthakur D (1996) Mimosine produced by the tree-legume Leucaena provides growth advantages to some Rhizobium strains that utilize it as a source of carbon and nitrogen. Plant Soil 186(1):87–92
Soedarjo M, Hemscheidt TK et al (1994) Mimosine, a toxin present in leguminous trees (Leucaena spp.), induces a mimosine-degrading enzyme activity in some Rhizobium strains. Appl Environ Microbiol 60(12):4268–4272
Suda S (1960) On the physiological properties of mimosine. Botanical Magazine 73(862):142–147
Tang SY, Ling KH (1975) The inhibitory effect of mimosine on collagen synthesis. Toxicon 13(5):339–342
Tangendjaja B, Lowry JB et al (1986) Isolation of a mimosine degrading enzyme from leucaena leaf. J Sci Food Agric 37(6):523–526
Tiwari MK, Moon HJ et al (2010) Cloning and characterization of a thermostable xylitol dehydrogenase from Rhizobium etli CFN42. Appl Microbiol Biotechnol 87(2):571–581
Waters JK, Karr DB et al (1985) Malate dehydrogenase from Rhizobium japonicum 3I1b-143 bacteroids and Glycine max root-nodule mitochondria. Biochemistry 24(23):6479–6486
Acknowledgments
We are thankful to Prof. E. J. Behrman (Dept. of Chemistry and Biochemistry, Ohio State University) for providing us the synthetic 3H4P. This research was supported by the National Science Foundation Award No. CBET 08-27057 and partially by a HATCH grant (CRIS 0216234). VSN was supported by IFP fellowship from the Ford Foundation for three years.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Negi, V.S., Bingham, JP., Li, Q.X. et al. midD-encoded ‘rhizomimosinase’ from Rhizobium sp. strain TAL1145 is a C–N lyase that catabolizes L-mimosine into 3-hydroxy-4-pyridone, pyruvate and ammonia. Amino Acids 44, 1537–1547 (2013). https://doi.org/10.1007/s00726-013-1479-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1479-z