Abstract
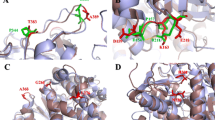
The β-glucosidases are enzymes essential for several industrial applications, especially in the field of plant structural polysaccharides conversion into bioenergy and bioproducts. In a recent study, we have provided a biochemical characterization of two hyperthermostable β-glucosidases from Thermotoga petrophila belonging to the families GH1 (TpBGL1) and GH3 (TpBGL3). Here, as part of a continuing investigation, the oligomeric state, the net charge, and the structural stability, at acidic pH, of the TpBGL1 and TpBGL3 were characterized and compared. Enzymatic activity is directly related to the balance between protonation and conformational changes. Interestingly, our results indicated that there were no significant changes in the secondary, tertiary and quaternary structures of the β-glucosidases at temperatures below 80 °C. Furthermore, the results indicated that both the enzymes are stable homodimers in solution. Therefore, the observed changes in the enzymatic activities are due to variations in pH that modify protonation of the enzymes residues and the net charge, directly affecting the interactions with ligands. Finally, the results showed that the two β-glucosidases displayed different pH dependence of thermostability at temperatures above 80 °C. TpBGL1 showed higher stability at pH 6 than at pH 4, while TpBGL3 showed similar stability at both pH values. This study provides a useful comparison of the structural stability, at acidic pH, of two different hyperthermostable β-glucosidases and how it correlates with the activity of the enzymes. The information described here can be useful for biotechnological applications in the biofuel and food industries.









Similar content being viewed by others
References
Aguilar CF, Sanderson I, Moracci M, Ciaramella M, Nucci R, Rossi M, Pearl LH (1997) Crystal structure of the β-glycosidase from the hyperthermophilic archeon Sulfolobus solfataricus: resilience as a key factor in thermostability. J Mol Biol 271:789–802
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The Swiss-model workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Bu L, Crowley MF, Himmel ME, Beckham GT (2013) Computational investigation of the pH dependence of loop flexibility and catalytic function in glycoside hydrolases. J Biol Chem 288:12175–12186
Cairns JRK, Esen A (2010) β-Glucosidases. Cell Mol Life Sci 67:3389–3405
Chi YI, Martinez-Cruz LA, Jancarik J, Swanson RV, Robertson DE, Kim SH (1999) Crystal structure of the beta-glycosidase from the hyperthermophile Thermosphaera aggregans: insights into its activity and thermostability. FEBS Lett 445:375–383
Colussi F, Garcia W, Rosseto FR, Mello BLS, Oliveira Neto M, Polikarpov I (2012) Effect of pH and temperature on the global compactness, structure, and activity of cellobiohydrolase Cel7A from Trichoderma harzianum. Eur Biophys J 41:89–98
Cota J, Alvarez TM, Citadini AP, Santos CR, Oliveira Neto M, Oliveira RR, Pastore GM, Ruller R, Prade RA, Murakami MT, Squina FM (2011) Mode of operation and low resolution structure of a multi-domain and hyperthermophilic endo-β-1,3-glucanase from Thermotoga petrophila. Biochem Biophys Res Commun 406:590–594
Cota J, Corrêa TLR, Damásio ARL, Diogo JA, Hoffmam ZB, Garcia W, Oliveira LC, Prade RA, Squina FM (2015) Comparative analysis of three hyperthermophilic GH1 and GH3 family members with industrial. New Biotechnol 32(1):13–20
Dias CA, Garcia W, Zanelli CF, Valentini SR (2013) eIF5A dimerizes not only in vitro but also in vivo and its molecular envelope is similar to the EF-P monomer. Amino Acids 44(2):631–644
Fischer H, de Oliveira Neto M, Napolitano HB, Polikarpov I, Craievich AF (2010) Determination of the molecular weight of proteins in solution from single small-angle X-ray scattering measurement on a relative scale. J Appl Cryst 43:101–109
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788
Gitlin I, Carbeck JD, Whitesides GM (2006) Why are proteins charged? Networks of charge-charge interactions in proteins measured by charge ladders and capillary electrophoresis. Angew Chem Int Ed Engl 45:3022–3060
Hall M, Rubin J, Behrens SH, Bommarius AS (2011) The cellulose-binding domain of cellobiohydrolase Cel7A from Trichoderma reesei is also a thermostabilizing domain. J Biotechnol 155:370–376
Jachimska B, Wasilewska M, Adamczyk Z (2008) Characterization of globular protein solutions by dynamic light scattering, electrophoretic mobility, and viscosity measurements. Langmuir 24:6866–6872
Jiang F, Kittle JD, Tan X, Esker AR, Roman M (2013) Effects of sulfate groups on the adsorption and activity of cellulases on cellulose substrates. Langmuir 29:3280–3291
Kado Y, Inoue T, Ishikawa K (2011) Structure of hyperthermophilic β-glucosidase from Pyrococcus furiosus. Acta Cryst F 67:1473–1479
Krissinel E, Henrick K (2007) Inference of macromolecular assemblies from crystalline state. J Mol Biol 372:774–797
Kumar R, Singh S, Singh OV (2008) Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol 35:377–391
Lombard V, Golaconda RH, Drula E, Coutinho PM, Henrissat B (2013) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42:D490–D495
Lundemo P, Adlercreutz P, Karlsson EN (2013) Improved transferase/hydrolase ratio through rational design of a family 1 β-Glucosidase from Thermotoga neapolitana. Appl Environ Microbiol 79:3400–3405
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577
Lynd LR, Laser MS, Bransby D, Dale BE, Davison B, Hamilton R, Himmel M, Keller M, McMillan JD, Sheehan J, Wyman CE (2008) How biotech can transform biofuels. Nat Biotechnol 26:169–172
McAndrew RP, Park JI, Heins RA, Reindl W, Friedland GD, D’haeseleer P, Northen T, Sale KL, Simmons BA, Adams PD (2013) From soil to structure, a novel dimeric β-glucosidase belonging to glycoside hydrolase family 3 isolated from compost using metagenomic analysis. J Biol Chem 288:14985–14992
Nakatani Y, Cutfield SM, Cowieson NP, Cutfield JF (2012) Structure and activity of exo-1,3/1,4-β-glucanase from marine bacterium Pseudoalteromonas sp. BB1 showing a novel C-terminal domain. FEBS J 279:464–478
Pozzo T, Pasten JL, Karlsson EN, Logan DT (2010) Structural and functional analyses of β-glucosidase 3B from Thermotoga neapolitana: a thermostable three-domain representative of glycoside hydrolase 3. J Mol Biol 397:724–739
Rezende CA, de Lima MA, Maziero P, Deazevedo ER, Garcia W, Polikarpov I (2011) Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels 4:54
Santiago PS, Carvalho FAO, Domingues MM, Carvalho JW, Santos NC, Tabak M (2010) Isoelectric point determination for Glossoscolex paulistus extracellular hemoglobin: oligomeric stability in acidic pH and relevance to protein-surfactant interactions. Langmuir 26:9794–9801
Santos CR, Squina FM, Navarro AM, Oldiges DP, Leme AF, Ruller R, Mort AJ, Prade R, Murakami MT (2011) Functional and biophysical characterization of hyperthermostable GH5 a-l-arabinofuranosidase from Thermotoga petrophila. Biotechnol Lett 33:131–137
Santos CR, Paiva JH, Meza AN, Cota J, Alvarez TM, Ruller R, Prade RA, Squina FM, Murakami MT (2012) Molecular insights into substrate specificity and thermal stability of a bacterial GH5-CBM27 endo-1,4-β-d-mannanase. J Struct Biol 177:469–476
Shewale JG (1982) β-Glucosidase: its role in cellulase synthesis and hydrolysis of cellulose. Int J Biochem 14:435–443
Silva VM, Colussi F, Oliveira Neto M, Braz A, Squina MF, Oliveira CL, Garcia W (2014) Modular hyperthermostable bacterial endo-β-1,4-Mannanase: molecular shape, flexibility and temperature-dependent conformational changes. Plos One 9(3):e92996
Sørensen A, Lübeck M, Lübeck PS, Ahring BK (2013) Fungal β-glucosidases: a bottleneck in industrial use of lignocellulosic materials. Biomolecules 3:612–631
Squina FM, Prade RA, Wang H, Murakami MT (2009) Expression, purification, crystallization and preliminary crystallographic analysis of an endo-1,5-α-l-arabinanase from hyperthermophilic Thermotoga petrophila. Acta Crystallogr Sect F 65:902–905
Suzuki K, Sumitani J, Nam Y, Nishimaki T, Tani S, Wakagi T, Kawaguchi T, Fushinobu S (2013) Crystal structures of glycoside hydrolase family 3 β-glucosidase 1 from Aspergillus aculeatus. Biochem J 452:211–221
Svergun DI (1992) Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J Appl Cryst 25:495–503
Svergun DI, Barberato C, Koch MHJ (1995) CRYSOL—a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J Appl Cryst 28:768–773
Takahata Y, Nishijima M, Hoaki T, Maruyama T (2001) Thermotoga petrophila sp. nov. and Thermotoga naphthophila sp. nov., Two hyperthermophilic bacteria from the Kubiki oil reservoir in Niigata Japan. Int J Syst Evol Microbiol 51:1901–1909
Tomovic A, Oakeley EJ (2008) Computational structural analysis: multiple proteins bound to DNA. PLoS One 3(9):e3243
Varghese JN, Hrmova M, Fincher GB (1999) Three-dimensional structure of a barley β-d-glucan exohydrolase, a family 3 glycosyl hydrolase. Structure 7:179–190
Xiao ZZ, Zhang X, Gregg DJ, Saddler JN (2004) Effects of sugar inhibition on cellulases and β-glucosidase during enzymatic hydrolysis of softwood substrates. Appl Biochem Biotechnol 113:1115–1126
Yoshida E, Hidaka M, Fushinobu S, Koyanagi T, Minami H, Tamaki H, Kitaoka M, Katayama T, Kumagai H (2010) Role of a PA14 domain in determining substrate specificity of a glycoside hydrolase family 3 β-glucosidase from Kluyveromyces marxianus. Biochem J 431:39–49
Zechel DL, Boraston AB, Gloster TM, Boraston CM, Macdonald JM, Tibrook DM, Stick RV, Davies GJ (2003) Iminosugar glycosidase inhibitors: structural and thermodynamic dissection of the binding of isofagomine and 1-deoxynojirimycin to β-glucosidases. J Am Chem Soc 125:14313–14323
Acknowledgments
We would like to thank the National Synchrotron Light Laboratory (LNLS, Brazil) and CENAPAD-SP (Centro Nacional de Processamento de Alto Desempenho em São Paulo). The authors would like to thank FAPESP and CNPq for the financial support for this work via grants # 2012/21054-9, # 478900/2012-0, # 2008/58037-9 and # 2011/13242-7; and fellowships # 2012/03503-0 (VMS) and # 501037/2012-8 (FC).
Conflict of interest
The authors declare that they do not have competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: C. Schiene-Fischer.
F. Colussi and V. M. da Silva have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Colussi, F., da Silva, V.M., Miller, I. et al. Oligomeric state and structural stability of two hyperthermophilic β-glucosidases from Thermotoga petrophila . Amino Acids 47, 937–948 (2015). https://doi.org/10.1007/s00726-015-1923-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-1923-3