Abstract
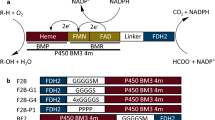
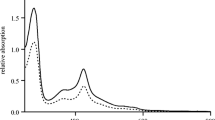
The human cytochrome P450s constitute an important family of monooxygenase enzymes that carry out essential roles in the metabolism of endogenous compounds and foreign chemicals. We present here results of a fusion between a human P450 enzyme and a bacterial reductase that for the first time is shown does not require the addition of lipids or detergents to achieve wild-type-like activities. The fusion enzyme, P450 2E1–BMR, contains the N-terminally modified residues 22–493 of the human P450 2E1 fused at the C-terminus to residues 473–1049 of the P450 BM3 reductase (BMR). The P450 2E1–BMR enzyme is active, self-sufficient and presents the typical marker activities of the native human P450 2E1: the hydroxylation of p-nitrophenol (K M=1.84±0.09 mM and k cat of 2.98±0.04 nmol of p-nitrocatechol formed per minute per nanomole of P450) and chlorzoxazone (K M=0.65±0.08 mM and k cat of 0.95±0.10 nmol of 6-hydroxychlorzoxazone formed per minute per nanomole of P450). A 3D model of human P450 2E1 was generated to rationalise the functional data and to allow an analysis of the surface potentials. The distribution of charges on the model of P450 2E1 compared with that of the FMN domain of BMR provides the ground for the understanding of the interaction between the fused domains. The results point the way to successfully engineer a variety of catalytically self-sufficient human P450 enzymes for drug metabolism studies in solution.






Similar content being viewed by others
Abbreviations
- δ-ALA:
-
δ-Aminolevulenic acid
- BMR:
-
Cytochrome P450 reductase domain of P450 BM3
- CPR:
-
Cytochrome P450 NADPH-dependent oxidoreductase
- DEAE:
-
(Diethylamino)ethyl
- DTT:
-
Dithiothreitol
- HPLC:
-
High-performance liquid chromatography
- IPTG:
-
Isopropyl-β-D-thiogalactopyranoside
- P450 BM3:
-
Bacterial cytochrome P450 from Bacillus megaterium
- P450 BMP:
-
Haem domain of P450 BM3
- P450 2E1:
-
Human cytochrome P450 2E1
- SRS:
-
Substrate recognition site
References
Poulos TL (1995) Cytochrome P450. Curr Opin Struct Biol 5:767–774
Nelson DR (1999) Cytochrome P450 and the individuality of species. Arch Biochem Biophys 369:1–15
Hasler JA, Estabrook RW, Murray M, Pikuleva I, Waterman M, Capdevila J, Holla V, Helvig C, Falck JR, Farrell G, Kaminsky LS, Spivack SD, Boitier E, Beaune P (1999) Human cytochrome P450. Mol Aspects Med 20:1–137
Guengerich FP (2001) Common and uncommon cytochrome P450 reactions related to metabolism and toxicity. Chem Res Toxicol 14:611–650
Nelson DR, Strobel HW (1988) On the membrane topology of vertebrate cytochrome P450 proteins. J Biol Chem 263:6038–6050
Guengerich FP, Kim DH, Iwasaki M (1991) Role of human cytochrome P450 2E1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol 4:168–179
Lieber CS (1997) Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev 77:517–538
Tanaka E, Terada M, Misawa S (2000) Cytochrome P450 2E1: its clinical and toxicological role. J Clin Pharm Ther 25:165–175
Chen W, Koenigs LL, Thompson SJ, Peter RM, Rettie AE, Trager WF, Nelson SD (1998) Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem Res Toxicol 11:295–301
Peter R, Bocker R, Beaune PH, Iwasaki M, Guengerich FP, Yang CS (1990) Hydroxylation of chlorzoxazone as a specific probe for human liver cytochrome P-450IIE1. Chem Res Toxicol 3:566–573
Koop DR (1986) Hydroxylation of p-nitrophenol by rabbit ethanol-inducible cytochrome P-450 isozyme 3a. Mol Pharmacol 29:399–404
Fantuzzi A, Fairhead M, Gilardi G (2004) Direct electrochemistry of immobilized human cytochrome P450 2E1. J Am Chem Soc 126:5040–5041
Narhi LO, Fulco AJ (1986) Characterization of a catalytically self-sufficient 119,000-Dalton cytochrome P-450 monooxygenase induced by barbituates in Bacillus megaterium. J Biol Chem 261:7160–7169
Narhi LO, Fulco AJ (1987) Identification and characterisation of two functional domains in cytochrome P-450BM-3, a catalytically self-sufficient monooxygenase induced by barbituates in Bacillus megaterium. J Biol Chem 262:6683–6690
Lewis DF, Hlavica P (2000) Interactions between redox partners in various cytochrome P450 systems: functional and structural aspects. Biochim Biophys Acta 1460:353–374
Boddupalli SS, Estabrook RW, Peterson JA (1990) Fatty acid monooxygenation by cytochrome P-450BM-3. J Biol Chem 265:4233–4239
Fang C, Koyabashi Y, Halpert JR (1997) Stoichometry of 7-ethoxycoumarin metabolism by cytochrome P450 2B1 wild-type and five active-site mutants. FEBS Lett 416:77–80
Mayuzumi H, Sambongi C, Hiroya K, Shimizu T, Tateishi T, Hatano M (1993) Effect of mutations of ionic amino acids of cytochrome P450 1A2 on catalytic activities toward 7-ethoxycoumarin and methanol. Biochemistry 32:5622–5628
Perret A, Pompon D (1998) Electron shuttle between membrane-bound cytochrome P450 3A4 and b5 rules uncoupling mechanisms. Biochemistry 37:11412–11424
Yun CH, Miller GP, Guengerich FP (2000) Rate-determining steps in phenacetin oxidations by human cytochrome P450 1A2 and selected mutants. Biochemistry 39:11319–11329
Noble MA, Miles CS, Chapman SK, Lysek DA, Mackay AC, Reid GA, Hanzlick RP, Munro AW (1999) Roles of key active-site residues in flavocytochrome P450 BM3. Biochem J 339:371–379
Munro AW, Leys DG, McLean KJ, Marshall KR, Ost TW, Daff S, Miles CS, Chapman SK, Lysek DA, Moser CC, Page CC, Dutton PL (2002) P450 BM3: the very model of a modern flavocytochrome. Trends Biochem Sci 27:250–257
Giannini S, Fairhead MJ, Gilardi G (2001) Engineering a soluble, catalytically self-sufficient human P450 for nanobiotechnology. Biochem Soc Trans 29:31
Gilardi G, Meharenna YT, Tsotsou GE, Sadeghi SJ, Fairhead M, Giannini S (2002) Molecular Lego: design of molecular assemblies of P450 enzymes for nanobiotechnology. Biosens Bioelectron 17:133–145
Fisher CW, Schet MS, Caule DL, Martin-Wintrom CA, Estabrook RW (1992) High-level expression in Escherichia coli of enzymatically active fusion proteins containing the domains of mammalian cytochromes p450 and NADPH-p450 reductase flavoprotein. Proc Natl Acad Sci USA 89:10817–10821
Shet MS, Fisher CW, Arlotto MP, Shakleton CHL, Holmans PL, Martin-Wixtrom CA, Saeki Y, Estabrook RW (1994) Purification and enzymatic properties of a recombinant fusion protein expressed in Escherichia coli containing the domains of bovine 17A and rat NADPH-P450 reductase. Arch Biochem Biophys 311:402–417
Shet MS, Fisher CW, Holmans PL, Estabrook RW (1993) Human cytochrome p450 3A4: enzymatic properties of a purified recombinant fusion protein containing NADPH-p450 reductase. Proc Natl Acad Sci USA 90:11748–11752
Harlow GR, Halpert JR (1996) Mutagenesis study of Asp-290 in cytochrome p450 2B11 using a fusion protein with rat NADPH-cytochrome p450 reductase. Arch Biochem Biophys 326:85–92
Lu P, Alterman MA, Chaurasia CS, Bambal RB, Hanzlick RP (1997) Heme-coordinating analogs of lauric acid as inhibitors of fatty acid ω-hydroxylation. Arch Biochem Biophys 337:1–7
Gilardi G, Meharenna YT, Tsotsou GE, Sadeghi SJ, Fairhead M, Giannini S (2002) Molecular Lego: design of molecular assemblies of P450 enzymes for nanobiotechnology. Biosens Bioelectron 17(1–2):133–145
Sadeghi SJ, Meharenna YT, Fantuzzi A, Valetti F, Gilardi G (2000) Engineering artificial redox chains by Molecular Lego. Faraday Discuss 116:135–153
Gillam EM, Guo Z, Guengerich FP (1994) Expression of modified human cytochrome P450 2E1 in Escherichia coli, purification, and spectral and catalytic properties. Arch Biochem Biophys 312:59–66
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver micrososmes. J Biol Chem 239:2370–2385
Ozols J, Strittmatter P (1964) The interaction of porphyrins and metalloporphyrins with apocytochrome b5. J Biol Chem 239:1018–1023
Yuan R, Madani S, Wei XX, Reynolds K, Huang SM (2002) Evaluation of cytochrome P450 probe substrates commonly used by the pharmaceutical industry to study in vitro drug interactions. Drug Metab Dispos 30:1311–1319
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Pontius J, Richelle J, Wodak SJ (1996) Quality assessment of protein 3D structures using standard atomic volumes. J Mol Biol 264:121–136
Vriend G (1996) WHAT IF: a molecular modeling and drug design program. J Mol Graph 8:52–56
Valetti F, Sadeghi SJ, Meharenna YT, Leliveld SR, Gilardi G (1998) Engineering multi-domain redox proteins containing flavodoxin as bio-transformer: preparatory studies by rational design. Biosens Bioelectron 13:675–685
Nishimoto M, Clark JE, Masters BSS (1993) Cytochrome P450 4A4: expression in Escherichia coli, purification, and characterisation of catalytic properties. Biochemistry 32:8863–8870
Gillam EMJ, Guo Z, Martin MV, Jenkins CM, Guengerich FP (1995) Expression of cytochrome P450 2D6 in Escherichia coli, purification, and spectral and catalytic characterization. Arch Biochem Biophys 319:540–550
Kery V, Elleder D, Kraus JP (1995) δ-Aminolevulinate increase heme saturation and yield of human cystathionine β-synthase expressed in Escherichia coli. Arch Biochem Biophys 316:24–29
Woodard SI, Dailey HA (1995) Regulation of heme biosynthesis in Escherichia coli. Arch Biochem Biophys 316:110–115
Koop DR (1990) Inhibition of ethanol-inducible cytochrome P450IIE1 by 3-amino-1,2,4-triazole. Chem Res Toxicol 3:377–383
Porter TD (1994) Mutagenesis at a highly conserved phenylalanine in cytochrome P450 2E1 affects heme incorporation and catalytic activity. Biochemistry 33:5942–5946
Larson JR, Coon MJ, Porter TD (1991) Alcohol-inducible cytochrome P-450IIE1 lacking the hydrophobic NH2-terminal segment retains catalytic activity and is membrane-bound when expressed in Escherichia coli. J Biol Chem 266:7321–7324
Dong JS, Porter TD (1996) Coexpression of mammalian cytochrome P450 and reductase in Escherichia coli. Arch Biochem Biophys 327:254–259
Zerilli A, Ratanasavanh D, Lucas D, Goasduff T, Dreano Y, Menard C, Picart D, Berthou F (1997) Both cytochromes P450 2E1 and 3A are involved in the O-hydroxylation of p-nitrophenol, a catalytic activity known to be specific for p450 2E1. Chem Res Toxicol 10:1205–1212
Chen W, Peter RM, McArdle S, Thummel KE, Sigle RO, Nelson SD (1996) Baculovirus expression and purification of human and rat cytochrome P450 2E1. Arch Biochem Biophys 335:123–130
Umeno M, McBride OW, Yang CS, Gelboin HV, Ginzalez FJ (1988) Human ethanol-inducible P450IIEI: complete gene sequence, promoter characterization, chromosome mapping and cDNA-directed expression. Biochemistry 27:9006–9013
Sevrioukova IF, Li H, Zhang H, Peterson JA, Poulos TL (1999) Structure of a cytochrome P450-redox partner electron-transfer complex. Proc Natl Acad Sci USA 96:1863–1868
Gotoh O (1992) Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J Biol Chem 267:83–90
Williams PA, Cosme J, Sridhar V, Johnson EF, McRee DE (2000) Mammalian microsomal cytochrome p450 monooxygenase: structural adaptations for membrane binding and functional diversity. Mol Cell 5:121–131
Gorsky LD, Koop DR, Coon MJ (1984) On the stoichiometry of the oxidase and monooxygenase reactions catalysed by liver microsomal P-450. J Biol Chem 259:6812–6817
Albano EA (1991) Role of ethanol inducible cytochrome p450 (p450IIE1) in catalyzing the free radicle activation of aliphatic alcohols. Biochem Pharmocol 41:1895–1902
Wang MH, Patten CJ, Yang GY, Paranawithana SR, Tan Y, Yang CS (1996) Expression and coupling of human cytochrome P450 2E1 and NADPH-cytochrome P450 oxidoreductase in dual expression and co-infection systems with baculovirus in insect cells. Arch Biochem Biophys 334:380–388
Patten CJ, Koch P (1995) Baculovirus expression of human P450 2E1 and cytochrome b5: spectral and catalytic properties and effect of b5 on the stoichiometry of P450 2E1-catalyzed reactions. Arch Biochem Biophys 317:504–513
Bridges A, Gruenke L, Chang YT, Vasker IA, Loew G, Waskell L (1998) Identification of the binding site on cytochrome P450 2B4 for cytochrome b5 & cytochrome P450 reductase. J Biol Chem 273:17036–17049
Helvig C, Capdevila JH (2000) Biochemical characterization of rat P450 2C11 fused to rat or bacterial NADPH-P450 reductase domains. Biochemistry 39:5196–5205
Davydov DR, Kariakin AA, Petushkova NA, Peterson JA (2000) Association of cytochromes P450 with their reductases: opposite sign of the electrostatic interactions in P450BM-3 as compared with the microsomal 2B4 system. Biochemistry 39:6489–6497
Zhao Q, Modi S, Smith G, Paine M, McDonagh PD, Wolf CR, Tew D, Lian LY, Roberts GC, Driessen HP (1999) Crystal structure of the FMN-binding domain of human cytochrome P450 reductase at 1.93 Å resolution. Protein Sci 8:298–306
Acknowledgements
We gratefully acknowledge Marcellus Ubbink for the gift of soluble bovine cytochrome b 5. This work was supported by a BBSRC studentship (UK), number 99/B1/E/05953, the Italian National Research Council (CNR) and Nano Biodesign Ltd (London).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fairhead, M., Giannini, S., Gillam, E.M. et al. Functional characterisation of an engineered multidomain human P450 2E1 by molecular Lego. J Biol Inorg Chem 10, 842–853 (2005). https://doi.org/10.1007/s00775-005-0033-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-005-0033-1