Abstract
Metal-hyperaccumulating plants have the ability to take up extraordinary quantities of certain metal ions without succumbing to toxic effects. Most hyperaccumulators select for particular metals but the mechanisms of selection are not understood at the molecular level. While there are many metal-binding biomolecules, this review focuses only on ligands that have been reported to play a role in sequestering, transporting or storing the accumulated metal. These include citrate, histidine and the phytosiderophores. The metal detoxification role of metallothioneins and phytochelatins in plants is also discussed.
Similar content being viewed by others
Introduction
Transition metals such as Mn, Fe, Ni, Cu and Zn are intrinsic components of enzymes and are therefore essential to plant and animal survival. Characteristic properties involved in transition metal selection include the high affinity of their ions for ligands containing nitrogen, oxygen and sulfur, plus access to different redox states in physiological conditions. This chemistry, however, also renders the metal ions extremely toxic at higher concentrations, as they can inhibit enzyme function and cause oxidative damage [1]. As well as controlling the concentrations of essential elements, plants also have to deal with the presence of nonessential metals and metalloids such as Cd, Hg, Pb, Se and As.
Hyperaccumulation
Some plants have evolved the ability to not only survive in metal-rich soils but to sequester and store exceptionally high levels of metals and metalloids in their above-ground tissues. These plants are called hyperaccumulators. There are four main types of metalliferous soils that host hyperaccumulators: serpentine soils derived from Fe- and Mg-rich ultramafic rocks that are enriched with Cr, Co and Ni; seleniferous soils derived from Se-rich rock types; calamine soils enriched with Zn, Cd and Pb; and Co- and Cu-containing soils derived from argillites and dolomites [2]. The most extreme example of hyperaccumulation reported to date is the Ni-hyperaccumulating tree from New Caledonia, Sebertia acuminata Pierre ex Baillon. The Ni concentration in the latex was determined to be ~25% of the dry mass (Fig. 1), contrasting with the usual value of <0.001% [3–5]. This is the highest concentration of Ni so far found in any biological fluid. The mass of Ni contained in one tree was estimated to be 37 kg [6]. Figure 1 also contains an image of Euphorbia helenae Urb. subspecies grandifolia Borhidi and Muñiz from Cuba. Green latex exudes when leaves are removed or branches broken. The Ni concentration in the dry mass of the exudate is usually around 3% [7].
Plants exhibit three basic responses when grown on soils with potentially toxic levels of metal ions: unrestricted uptake, exclusion and (hyper)accumulation (Fig. 2) [8, 9]. Uncontrolled uptake of metal ions from soils normally results in toxicity and death. Excluders do not translocate metal ions to the above-ground tissues, a trait which enables them to grow on soils toxic to most plants. Hyperaccumulators often exhibit higher metal concentrations in their tissues than are present in the soil or hydroponic solution and can tolerate much higher metal concentrations before showing symptoms of toxicity. In the serpentine-endemic shrub Alyssum lesbiacum (Candargy) Rech. from Lesbos (Greece), Ni was detected at millimolar concentrations in xylem sap, accumulated from micromolar levels of Ni in hydroponic solution [10, 11].
Hyperaccumulation has been recognized as an extreme physiological response in heavy metal tolerance [8]. This trait allows these plants to respond positively to the presence of their characteristic metal ions. When grown in soils with normal metal concentrations, hyperaccumulator species sequester much higher metal concentrations in their shoots than do normal plants [12, 13]. However, when transplanted to soil containing low levels of these ions, hyperaccumulators display normal growth patterns, indicating that there is no essential physiological requirement for the high metal concentrations [14]. It has been suggested that metal hyperaccumulation provides protection from fungal and herbivore attack [15–18]. This has been observed serendipitously in glasshouse experiments carried out by the authors (Fig. 3). An absence of Ni in the Ni hyperaccumulator Thlaspi goesingense Halácsy increased susceptibility to powdery mildew (Erysiphe cruciferarum Opiz ex L. Junell) [19].
a The two Berkheya coddii Roessler plants on the right were grown on serpentine soils resulting in high Ni concentrations in the leaves. The two plants on the left were grown in potting mix, resulting in a low Ni concentration. Insect attack was much reduced for the plants grown on serpentine soil; b close-up of leaves, left low Ni; right high Ni
The physiological processes involved in hyperaccumulation are not well understood. Plants must be able to store the metal ions in nonlabile complexes to eliminate toxic effects. The most likely areas for storage are the cell wall, the cytosol and the vacuole. A number of steps are required for metal ions to reach the storage tissues: mobilization and uptake from soil, compartmentation and sequestration within roots, transfer to the xylem for transport, distribution between metal sinks in above-ground tissue and sequestration and storage in leaf cells [20]. Each stage could affect metal accumulation. Hyperaccumulators also need to combat the damaging effects of high metal concentrations. For example, increased glutathione production in Thlaspi Ni hyperaccumulators appears to reduce the damaging oxidative effects of high Ni concentrations [21].
Metal uptake and transport strategies
Plant ligands play a role in the sequestration of metals from soils, transport to the above-ground tissue and finally storage. The first stage of metal uptake in plants is the interaction with the soil. When provided as common inorganic salts, the essential transition elements, particularly Fe, are relatively insoluble in soils and in nutrient solutions. In general, solubility decreases markedly at pH >5 as a result of the formation of insoluble hydrous oxides (such as rust). In order to solubilize metals for absorption, plants need to interact with the soil in the rhizosphere (soil zone immediately surrounding the root). Increased availability appears to be a consequence of factors such as the presence of microbes, a decrease in pH, a change in the redox potential and/or the exudation of ligands [22, 23]. Two strategies have been identified [24]. In strategy I, the plants (dicotyledons and non-grass monocotyledons) solubilize Fe(III) by reduction to the more soluble Fe(II) and decrease pH by H+ excretion. A 1000-fold increase in solubility occurs for every unit decrease in pH [25]. Fe(II) is then absorbed directly by the roots. In strategy II, the plants (graminaceous monocotyledons) produce specific ligands (phytosiderophores, PS) from the mugineic acid family. These are secreted from the roots and coordinate Fe(III) in the rhizosphere. The Fe(III)–PS complexes are then absorbed by the roots [26]. In T. goesingense, increased Ni(II) mobilization in the rhizosphere has been linked to exudation of ligands [27]. Relative to the bulk soil concentrations, water extracts derived from the T. goesingense rhizosphere contained increased levels of Ni and dissolved carbon species. The levels were also higher than those observed for the Ni excluder species in the same soil. However, related studies on the same plant provided contradicting data: metal mobilization studies found that root exudates did not significantly enhance the release of Ni, Zn or Cd under the specific conditions examined [28, 29].
The movement of metal ions to the above-ground tissue is the next important stage of transport. It has been suggested that hyperaccumulation may be a result of the evolution of a particularly effective metal translocation pathway from roots to shoots. This appears to be true for Zn hyperaccumulation in T. caerulescens J. and C. Presl [30]. A comparative study on the Ni hyperaccumulator T. goesingense and the nonaccumulator T. arvense L. found that the net root-to-shoot translocation of Ni appeared to be very similar in each plant for the first 4–5 days of Ni exposure. After this period, it was concluded that Ni toxicity stalled translocation in the nonaccumulator while the hyperaccumulator continued to be healthy, apparently being able to detoxify the metal at a cellular level [31]. The hyperaccumulator may be detoxifying the metal in the leaves via strong binding ligands.
Plant ligands
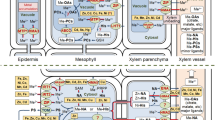
Only a small fraction of the metal content in plants is present in the form of free aqua ions. It is assumed that most ions are bound to low molecular mass ligands or to proteins [32]. The fact that hyperaccumulators can amass metal concentrations of greater that 1% dry mass implies the existence of a metal detoxification mechanism that involves selective ligands. Plants produce a number of possible ligands, including organic acids, amino acids, peptides and proteins. A selection of proposed ligands is displayed in Fig. 4. Other organisms are known to use specific proteins (chaperones) as intracellular ion carriers that deliver metals to specific destinations. For example, Cu is a cofactor in many enzymes but “free” Cu (as Cu+ or Cu2+) will damage biomolecules by adventitious binding or by producing radicals. In yeast, it has been shown that intracellular Cu concentrations are tightly controlled by Cu chaperones [33].
This review focuses on plant ligands that have been shown to play a role in hyperaccumulation and examines the structure of metal-ion complexes identified in hyperaccumulating plants. It is important to consider the association constants of possible metal-ion complexes. For example, those for the proposed ligand nicotianamine (NA) are very high while those of organic acids (such as citrate) are so low that selective formation of nonlabile complexes for a given metal in plants is unlikely (Table 1). This is a strong argument against a role for the latter acids in the hyperaccumulation mechanism. They may play a part in sequestration within isolated compartments.
Histidine
Nitrogen-donor ligands and especially free amino acids are assumed to play a role in hyperaccumulators [11]. Among them, histidine (His) is considered to be the most important free amino acid involved in metal hyperaccumulation. It can act as a tridentate ligand via its carboxylato, amine and imadazole functions (for example, NiII(His)2, Figs. 4, 5) [34]. However, it is a versatile ligand with a number of known coordination modes. For example, the complex CuII(His)2, a component of mammalian blood, features a five-coordinate site with one tridentate and one bidentate ligand [35].
Crystal structure of NiII(His)2 (Ni in pink) [34]
Histidine has a relatively high association constant for Ni2+ (Table 1). Spectroscopic data consistent with the presence of NiII-His complexes has been reported for hyperaccumulating plants [32]. Exposure of the hyperaccumulator A. lesbiacum to Ni elicited a large and proportional increase in the levels of free His (Fig. 6). This effect was not seen in the nonaccumulator Alyssum montanum L. [11]. Treatment of the latter with His (as a foliar spray or by addition to the root medium) greatly increased Ni tolerance, as well as the capacity for Ni transport to the shoots [36]. This was also demonstrated for the pea Pisum sativum L. [37]. In the Indian mustard Brassica juncea (L.) Czern. (a nonaccumulator), Ni mobility from root to shoot was increased upon exogenous addition of His [38]. While enhanced tolerance to the Ni2+ ion followed an increase in the rate of biosynthesis of free His in Arabidopsis thaliana (L.) Heynh., this did not increase accumulation of Ni in the shoots [39]. Ni treatment of roots or shoots of T. goesingense did not lead to an increase in the mRNA levels of genes involved in His biosynthesis [40]. However, transcription levels of seven of the eight enzymes in this pathway were higher in the Ni hyperaccumulator species A. lesbiacum than those in the nonaccumulator A. montanum. The first enzyme in the pathway, ATP-phosphoribosyltransferase, showed the largest increase. Overexpression of this enzyme in transgenic Arabidopsis thaliana increased tolerance to Ni, although concentrations of Ni detected in the xylem sap and shoot tissue did not change [41].
Changes in the amino acid composition of xylem sap as a response to nickel exposure in the root medium [11]
X-ray absorption spectroscopy (XAS) examinations of root and shoot tissue from T. goesingense and the nonaccumulator species T. arvense revealed no major differences in the coordination of Ni by His [40]. This technique cannot discriminate easily between second row ligand atoms N and O. However, given sufficient signal intensity in the extended X-ray absorption fine structure (EXAFS) associated with the Ni XAS spectrum, His can be identified by a specific multiple scattering pattern when bound via the imidazole ring. Overall, these results suggest that His is involved in the hyperaccumulation of Ni(II) for some, but not all, of the species examined. However, its detailed role remains to be defined.
Other XAS studies on T. caerulescens roots detected Zn bond lengths typical of Zn–O, N suggesting coordination by His [32]. However, at present such sites cannot be distinguished from Zn 2+aq .
Organic acids
The carboxylic acids known to be present in high concentrations in the cell vacuoles of photosynthetic tissues include citric, isocitric, oxalic, tartaric, malic, malonic and aconitic [42]. Many studies have implied that these acids play a role in hyperaccumulation [36, 43–50]. The roots of Arabidopsis halleri (L.) O’Kane and Al-Shehbaz showed an increase in the levels of organic acids in response to increasing Zn levels in the soil [51]. The application of metal XAS to hyperaccumulators has detected O- or N-based ligands, consistent with the presence of the organic acid ligands mentioned above. Analysis of the extended EXAFS of the XAS spectrum for T. caerulescens suggested that the coordination environment of Cd/Zn centres varies with tissue age. In mature and senescent leaves, metal–O bonds appeared to dominate. It was suggested that this plant prefers to detoxify metals by pumping them into vacuoles rather than by synthesizing specific ligands [52]. However, in younger tissues, a higher percentage of Cd appeared to be bound by sulfur ligands (such as phytochelatins; vide infra) and a higher percentage of Zn by His. This may indicate that younger tissues require stronger ligands such as those containing N and S for metal detoxification, in addition to detoxification by sequestration by organic acids in the epidermal cell vacuoles. However, the apparent absence of Zn–S bonds suggested that sulfur ligands are not involved in Zn resistance in T. caerulescens [52]. Localisation studies on hyperaccumulators have not detected Ni associated with S ligands [28, 31].
Analysis of the Ni-rich latex of S. acuminata indicated that most of the Ni is bound to citrate (Figs. 1, 4) [3, 6, 53, 54]. The EXAFS of the XAS spectrum from leaves (containing 2.5% dry weight Ni) also suggested that Ni may be bound to citrate in the cell vacuoles [5]. As with Zn, S-containing ligands such as phytochelatins do not appear to be involved in nickel detoxification [6].
A number of roles have been inferred for organic acids (particularly citrate) in the process of hyperaccumulation. It is likely that their function varies from species to species. Although organic acids are found in high concentrations in plants, they do not bind metal ions strongly enough to extract them from soils (Table 1) or to induce the specificity observed in many hyperaccumulators. They may play a role in sequestering metals ions in the vacuoles but are unlikely to act as long-distance transporters.
Phytosiderophores: nicotianamine
Nicotianamine is the precursor for a group of multidentate plant ligands in graminaceous plants known as phytosiderophores (PS; examples include mugineic acid and avenic acid; Fig. 4). It is formed by the condensation of three S-adenosyl-methionine molecules (SAM), catalysed by the enzyme nicotianamine synthase (NAS; Scheme 1) [55, 56]. Subsequently, nicotianamine aminotransferase (NAAT) catalyses the conversion of NA to mugineic acid derivatives (Scheme 1) and is present only in graminaceous (strategy II) plants [24]. The products are converted to a range of potential ligands featuring alternating amino and carboxylato groups. NA was first isolated from the leaves of the tobacco plant Nicotiana tabacum L., in 1971 [57], and has been subsequently found in all naturally occurring multicellular plants. Its identity was first established by Kristensen [58]. There are six alternating carboxylate and amino groups whose relative positions favour the formation of six-coordinate metal complexes. Association constants almost match those of the classic ligand ethylenediaminetetraacetic acid (EDTA; Table 1). Although crystal structures of metal–NA complexes are not available, complexes are assumed to be hexadentate and similar in structure to the CuII-mugineic acid complex [CuII(C12H18N2O8)]- (Fig. 7) [59].
CuII-mugineic acid complex [CuII(C12H18N2O8)]- (Cu in pink) [59]
Nicotianamine is found throughout the plant in varying concentrations and levels can reach up to 400 μmol/g fresh mass in growing tissues [60]. It appears to be linked to Fe homeostasis. When grown in Fe-deficient conditions, barley plants exhibited a fivefold increase of NAS activity [61]. Takahashi et al. [62] introduced the gene that encodes NAAT into the nongraminaceous plant tobacco. Its expression led to a deficiency in NA (see Scheme 1) and induced abnormal phenotypes, apparently a result of deficiencies in internal metal transport. Lycopersicon esculentum Mill. c.v. chloronerva, an NA auxotroph, exhibits Fe-deficiency symptoms, such as excessive root branching, intercostal chlorosis, retarded growth and irregular Fe distribution in young growing tissues. Fe accumulates within the roots of c.v. chloronerva, suggesting that it cannot be mobilized and distributed to above-ground tissue [63]. Exogenous addition of NA to c.v. chloronerva restored growth, development and chlorophyll synthesis and was transported between different organs [64]. When fed to either the leaves or roots, NA was subsequently found in other parts of the plant, implying a ready transport mechanism [65]. NA may also be important in Cu translocation as NA levels increased in tomato plants exposed to high levels of Cu [66, 67].
Nicotianamine appears to be required for plant fertility. It may transport metals to cells of the reproductive organs through a specific NA–metal transporter in vascular bundles of young leaves, flowers and seeds [62]. In cases where Fe(II) reaches toxic levels, NA may act as an Fe(II) scavenger to protect cells from oxidative stress [68]. An increase in NA production was observed when Fe was overloaded in Fe-accumulating pea mutants [69].
Most research on PSs in plants has been focused on Fe transport [26]. In addition, it has been proposed that NA may be involved in the complexation of metal ions as micronutrients in both hyperaccumulators and nonhyperaccumulators [60, 70–73]. It has been suggested that higher NAS activity is a key factor in Zn tolerance and hyperaccumulation [70, 74]. A similar situation may be true with Ni. An NAS gene of Arabidopsis thaliana was ectopically expressed in transgenic tobacco plants. These plants were grown in media with elevated concentrations of Mn, Ni, Cu and Cd. A difference between overexpressing and wild-type plants was only seen with Ni. The increased NA concentration enhanced Ni tolerance in all of the transgenic plants at phytotoxic concentrations up to 1 mM. However, Ni uptake rates were not increased [75]. Complexes of NA with Ni were identified by mass spectrometry in extracts of the Ni hyperaccumulating plants T. caerulescens and S. acuminata [53, 72]. It is not surprising that Ni–NA was detected under such conditions as NA binds Ni2+ even more strongly than Fe2+ (Table 1), in accordance with the Irving–Williams series [76].
Synthetic nicotianamine analogues
Synthesis of NA and other PSs was achieved by coupling peptide intermediates with selective amide reduction [77–81]. Structural analogues and isomers of NA have been isolated (see for example Fig. 8) and their ability to bring about phenotypical normalization in the NA auxotroph c.v. chloronerva has been examined [82, 83]. Root elongation was used as a measure of biological activity as well as the greening of chlorotic intercostal areas of young leaves. The NA analogues studied exhibited lower association constants than the naturally occurring S,S,S stereoisomer [83]. The reduced affinity of the R,S,S isomer was attributed to a strained chair conformation within one of the six-member rings. Despite the lower association constants, some of these analogues elicited similar responses to those observed with NA. For example, hexadentate NA analogues induced significant increase in root growth and greening of chlorotic leaf areas. This suggests that stereospecific binding to sites such as membranes or receptor proteins for NA are not important and that NA acts as a complexing agent. Such an interpretation is at odds with that of Takahashi et al. [62] who suggested that there is a specific transporter protein for NA–metal complexes. These researchers also demonstrated that citrate could not substitute for NA in the transport of metal ions in young leaves and flowers. These findings are not surprising in the sense that the association constants for citrate binding to transition metal ions are much lower than those of the corresponding NA complexes (Table 1).
Metallothioneins and phytochelatins
The cysteine-rich metallothionein proteins (MT; molar mass <10 kDa) have been identified in plants, animals, eukaryotic microorganisms and certain prokaryotes [71, 84]. While their exact function remains a matter of debate, they appear to play a role in metal tolerance and/or homeostasis. They bind between six and eight Cu+, Zn2+, Cd2+, Hg2+ and Pb2+ ions as M3 and M4 clusters. Thiolato sulfur atoms of cysteinyl sidechains act as bridging ligands in the clusters [85]. The molecular structure of a Cu I8 centre resolved in a yeast Cu–MT has been interpreted in terms of a safe depository for toxic Cu+ ions which may also allow delivery of Cu to transport proteins [86]. The specific functions of plant MTs are not clear as they belong to a diverse family of genes [87]. A positive correlation was found between MT gene expression and Cu tolerance in a number of Arabidopsis populations [88]. However, the role of MT in hyperaccumulators is not understood.
Phytochelatins (PCs) are small cysteine-rich peptides which clearly have a role in metal detoxification in plants [89]. PCs normally contain only three amino acids: glutamic acid (Glu), cysteine (Cys), and glycine (Gly). The general formula is (GluCys)nGly (n=2–11) with Glu bound to Cys through γ-peptide linkages (Fig. 4). There are also a number of structural variants [90]. Although PCs are structurally similar to MTs, they are synthesized enzymatically while MTs are encoded by genes. Following Cd exposure, PCs have been found in all plants examined. This includes vascular and nonvascular (mosses, algae) plants and plants growing in their natural habitats in soils with elevated metal concentrations [71, 91]. PCs were also produced in the presence of elevated levels of Zn and Cu, suggesting a role in the detoxification of these essential elements. In addition, production of PCs has been shown to be induced at varying levels by a wide range of metal ions. The most effective appeared to be Ag, As, Cd, Hg, and Pb ions [92].
The sensitivities of a number of hyperaccumulators and nonhyperaccumulators to Co, Ni, Cu and Zn were unaffected by treatment with an inhibitor of γ-glutamylcysteine synthetase, an enzyme essential for PC synthesis [90]. Cd sensitivity was increased only in plants (Silene vulgaris (Moench) Garcke) lacking Cd tolerance [90]. Consequently, the mechanism for hyperaccumulation for the metals mentioned above may not be dependent on PC production. Similar observations were made by Ebbs et al. [93]. Overexpression of an Arabidopsis PC synthase in transgenic Arabidopsis showed hypersensitivity to Zn and Cd but not to Cu. The transgenic lines were more sensitive to Cd than a PC-deficient Arabidopsis mutant. It was suggested that the Cd hypersensitivity was due to the toxicity of PCs when present above optimal levels [94]. PC-deficient mutants of Arabidopsis thaliana and the yeast Schizosaccharomyces pombe showed hypersensitivity to Cd and As but lower sensitivity to metals such as Cu [95].
Sensitivity to As has been observed in both As-tolerant plants and in nonaccumulators when PC synthesis was inhibited [90]. Studies on the As hyperaccumulators Pteris vittata L. (Chinese brake fern) and P. cretica also indicated that PCs play a role in the sequestration of As [96–99]. XAS data from the leaves of P. vittata indicated that, at extremely high As concentrations, 94% was present as aqueous As(III) with the remaining 6% bound to sulfur ligands [100]. These data contrast with those from an XANES study on the nonaccumulator B. juncea which was interpreted in terms of 95% of As(III) being coordinated by S in a tris(thiolato)arsenic(III) complex [101].
From the evidence cited above, a distinction can be made between normal and hyperaccumulating plants as far as PCs are concerned. Nonaccumulating plants coordinate the majority of Cd and As using PCs and hyperaccumulators do not. There is no evidence that PCs play a role in Zn or Ni hyperaccumulation.
Summary
Hyperaccumulators generally show selectivity for a particular metal ion. For example, the Alyssum Ni hyperaccumulators exhibit Ni:Co selectivity in the range 100–5,000:1. Co uptake only occurs when this element is provided in soluble form to a soil of low Ni content [2]. An understanding of the molecular basis of this selectivity would be interesting from an industrial point of view considering that the chemical and physical properties of Ni2+ and Co2+ are similar. The differences in the association constants of the Ni and Co complexes with NA and other plant ligands such as citrate or His are not large enough (Table 1) to explain the selective accumulation observed. Selective Ni/Co transporters are widely found among bacteria and certain fungi [102]. Metal accumulation assays on various Ni/Co transport proteins (NiCoT) in E. coli have identified subtypes with differing selectivities. These range from strict transport of Ni or Co only to nonselective transport of both ions. Some information is available on the domains responsible for these varying properties. For example, replacement of the first His residue of the NiCoT from Cupriavidus necator reduced the affinity for Ni2+ [103]. The same variation to the NiCoT of Rhodococcus rhodochrous reduced affinity for both Ni2+ and Co2+ [103]. Similar selection mechanisms may be present in the hyperaccumulators.
Nonselective behaviour has been observed. In the genus Thlaspi, several species can accumulate multiple elements. T. caerulescens can accumulate Mn, Co, Ni, Zn and Cd, suggesting that a common mechanism of hyperaccumulation for several metals may operate in this plant [104].
Nicotianamine may play a role in hyperaccumulation as a long-distance transporter. However, it is insufficiently selective to be involved in metal ion selection. As for most low molecular mass ligands, selectivity is determined by the Irving–Williams series. Discrimination could occur in the root (via a plasma membrane transport protein such as the transporters mentioned above), during xylem loading and transport or upon transfer to storage tissues. Binding to organic acids cannot account for the specificity. Specific ligands yet to be characterised may be responsible for the specificity of metal ion hyperaccumulators. NA is a good example of a low molecular mass ligand which appears to be involved in Fe homeostasis. There may be similar mechanisms involved in hyperaccumulation. For example, an XAS study of tissue from Datura innoxia Miller exposed to varying Zn concentrations indicated the presence of ZnII(O,N)n centres (n=4 or 5). Estimated bond lengths were significantly shorter than those expected for typical Zn–O, N or Zn–N(His) complexes, suggesting that a novel Zn-binding protein may be involved in detoxification [105].
Authors’ perspective
Methods for metal ion speciation analysis have been reviewed by Szpunar [106, 107]. Significant difficulties arise with sampling. Ideally, the metal ion complexes should be isolated without disturbing the chemical equilibria within the cells. Release of cell contents upon disruption might induce formation of complexes not present in vivo. Surface functional groups on proteins can also bind metals. Careful isolation of metal ion complexes is required when using techniques such as mass spectrometry.
Molecular biology and in particular gene-based studies have the potential to circumvent the sampling problems mentioned above. Genes that confer heavy metal tolerance can be identified and expressed. For example, the heavy metal transporter ZnT-1 has been shown to mediate Zn2+ uptake and is expressed at high levels in both the roots and shoots in T. caerulescens [108]. High transcription levels for ZnT-1 correlate with an increased rate of Zn influx in this plant.
New techniques allowing detailed cellular studies are currently being developed. Imaging mass spectrometry combines molecular information, including relative molecular abundances, with the spatial distribution of a microscope. This results in a unique element/molecule-specific map at submicron spatial resolution. Synchrotron-based techniques also allow element-specific imaging. These techniques will provide new information concerning the high concentrations of metal ions stored in hyperaccumulators.
An understanding of the remarkable selectivity achieved by metal hyperaccumulators will be beneficial to a number of fields including phytoremediation (remediation of contaminated soils using plants), crop improvement and phytomining. There are plants which are known to remove Ni, Zn, Cd, As and Se at rates which offer viable remediation solutions [109]. The identification of highly selective ligands could be applied to the industrial separation of metals. While hyperaccumulators appear to offer solutions in a number of areas, a better understanding of the metal accumulation pathways is needed for the full potential of this unique plant feature to be utilized.
Abbreviations
- Ala:
-
Alanine
- Arg:
-
Arginine
- Asn:
-
Asparagine
- Asp:
-
Aspartic acid
- Cys:
-
Cysteine
- EXAFS:
-
Extended X-ray absorption fine structure spectroscopy
- Gln:
-
Glutamine
- Glu:
-
Glutamic acid
- Gly:
-
Glycine
- His:
-
Histidine
- Ile:
-
Isolucine
- Leu:
-
Leucine
- Lys:
-
Lysine
- MT:
-
Metallothionein
- NA:
-
Nicotianamine
- NAAT:
-
Nicotianamine aminotransferase
- NAS:
-
Nicotianamine synthase
- NiCoTs:
-
Ni/Cobalt transporters
- PC:
-
Phytochelatin
- Phe:
-
Phenylalanine
- Pro:
-
Proline
- PS:
-
Phytosiderophores
- SAM:
-
S-adenosyl-methionine
- Ser:
-
Serine
- Thr:
-
Threonine
- Tyr:
-
Tyrosine
- Val:
-
Valine
- XAS:
-
X-ray absorption spectroscopy
References
Anderson C, Deram A, Petit D, Brooks R, Stewart R, Simcock R (2001) In: Iskandar IK, Kirkham MB (eds) Trace elements in soil: bioavailability, flux, and transfer. Lewis, Washington DC, pp 63–76
Reeves RD, Baker AJM (2000) In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals. Wiley, New York, pp 193–229
Jaffré T, Brooks RR, Lee J, Reeves RD (1976) Science 193:579–580
Bollard EG (1983) In: Lauchli A, Bielsky RL (eds) Inorganic plant nutrition (Encyclopedia of plant physiology, vol 15B). Springer, Berlin Heidelberg New York, pp 695–744
Perrier N, Colin F, Jaffré T, Ambrosi J, Rose J, Bottero J (2004) CR Geoscience 336:567–577
Sagner S, Kneer R, Wanner G, Cosson JP, Deus-Neumann B, Zenk MH (1998) Phytochemistry 47:339–347
Reeves RD, Baker AJM, Borhidi A, Berazain R (1996) New Phytol 133:217–224
Baker AJM (1981) J Plant Nutr 3:643–654
McGrath SP, Dunham SJ, Correll RL (1999) In: Terry N, Bañuelos GS (eds) Phytoremediation of contaminated soil and water. Lewis, Boca Raton, FL, pp 109–128
Salt DE, Krämer U (2000) In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals. Wiley, New York, pp 231–246
Krämer U, Cotter-Howells JD, Charnock JM, Baker AJM, Smith JAC (1996) Nature 379:635–638
Brown SL, Chaney RL, Angle JS, Baker AJM (1994) J Environ Qual 23:1151–1157
McGrath SP, Shen ZG, Zhao FJ (1997) Plant Soil 188:153–159
Reeves RD, Baker AJM (1984) New Phytol 98:191–204
Hanson B, Lindblom SD, Loeffler ML, Pilon-Smits EAH (2004) New Phytol 162:655–662
Boyd RS (1998) In: Brooks RR (ed) Plants that hyperaccumulate heavy metals. CAB Int., Oxford, pp 181–201
Mesjasz-Przybylowicz J, Przybylowicz WJ (2001) S Afr J Sci 97:596–598
Davis MA, Boyd RS, Cane JH (2001) S Afr J Sci 97:554–557
Freeman JL, Garcia D, Kim D, Hopf A, Salt DE (2005) Plant Physiol 137:1082–1091
Clemens S, Palmgren MG, Krämer U (2002) Trends Plant Sci 7:309–315
Freeman JL, Persans MW, Nieman K, Albrecht C, Peer W, Pickering IJ, Salt DE (2004) Plant Cell 16:2176–2191
Whiting SN, de Souza MP, Terry N (2001) Environ Sci Technol 35:3144–3150
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Römheld V, Marschner H (1986) Plant Physiol 80:175–180
Rengel Z (1999) In: Prasad MNV, Hagemeyer J (eds) Heavy metal stress in plants. Springer, Berlin Heidelberg New York, pp 231–251
Reichman SM, Parker DR (2005) In: Huang PMG, G (ed) Biogeochemistry of trace elements in the rhizosphere. Elsevier, Toronto
Wenzel WW, Bunkowski M, Puschenreiter M, Horak O (2003) Environ Pollut 123:131–138
Zhao FJ, Hamon RE, McLaughlin MJ (2001) New Phytol 151:613–620
Salt DE, Kato N, Krämer U, Smith RD, Raskin I (2000) In: Terry N, Bañuelos GS (eds) Phytoremediation of contaminated soil and water. Lewis, Boca Raton, FL, pp 189–200
Lasat MM, Baker AJM, Kochian LV (1996) Plant Physiol 112:1715–1722
Krämer U, Smith RD, Wenzel WW, Raskin I, Salt DE (1997) Plant Physiol 115:1641–1650
Salt DE, Prince RC, Baker AJM, Raskin I, Pickering IJ (1999) Environ Sci Technol 33:713–717
Finney LA, O’Halloran TV (2003) Science 300:931–936
Fraser KA, Long HA, Candlin R, Harding MM (1965) Chem Comm 15:344–345
Deschamps P, Kulkarni PP, Gautam-Basak M, Sarkar B (2005) Coord Chem Rev 249:895–909
Krämer U, Pickering IJ, Prince RC, Raskin I, Salt DE (2000) Plant Physiol 122:1343–1353
Kameda Y, Ito E, Takeshita A, Nakazawa R, Takenaga H (2002) Tokyo Nogyo Daigaku Nogaku Shuho 47:45–48
Kerkeb L, Krämer U (2003) Plant Physiol 131:716–724
Wycisk K, Kim EJ, Schroeder JI, Krämer U (2004) FEBS Lett 578:128–134
Persans MW, Yan X, Patnoe J-MML, Krämer U, Salt DE (1999) Plant Physiol 121:1117–1126
Ingle RA, Mugford ST, Rees JD, Campbell MM, Smith JAC (2005) Plant Cell 17:2089–2106
Terry N, Bañuelos GS (1999) Phytoremediation of contaminated soil and water. Lewis, Boca Raton, FL
Sarret G, Saumitou-Laprade P, Bert V, Proux O, Hazemann J-L, Traverse A, Marcus Matthew A, Manceau A (2002) Plant Physiol 130:1815–1826
Qiu R-L, Tang Y-T, Fang X-H, Chaney RL, Li Y-M, Angle JS, Liu W, Zeng X-W (2004) Zhongshan Daxue Xuebao, Ziran Kexueban 43:144–149
de la Rosa G, Peralta-Videa JR, Montes M, Parsons JG, Cano-Aguilera I, Gardea-Torresdey JL (2004) Chemosphere 55:1159–1168
Shan X, Wang H, Zhang S, Zhou H, Zheng Y, Yu H, Wen B (2003) Plant Sci 165:1343–1353
de la Rosa G, Gardea-Torresdey JL, Peralta-Videa JR, Parsons JG, Montes M (2003) ACS National Meeting, Division of Environmental Chemistry 43:601–606
Boominathan R, Doran PM (2003) J Biotechnol 101:131–146
Bidwell SD, Woodrow IE, Batianoff GN, Sommer-Knudsen J (2002) Funct Plant Biol 29:899–905
Memon AR, Yatazawa M (1984) J Plant Nutr 7:961–974
Zhao FJ, Lombi E, Breedon T, McGrath SP (2000) Plant Cell Environ 23:507–514
Küpper H, Mijovilovich A, Meyer-Klaucke W, Kroneck Peter MH (2004) Plant Physiol 134:748–757
Schaumlöffel D, Ouerdane L, Bouyssiere B, Lobinski R (2003) J Anal Atom Spectrom 18:120–127
Lee J, Reeves RD, Brooks RR, Jaffré T (1978) Phytochemistry 17:1033–1035
Shojima S, Nishizawa NK, Fushiya S, Nozoe S, Kumashiro T, Nagata T, Ohata T, Mori S (1989) Biol Met 2:142–145
Higuchi K, Kanazawa K, Nishizawa N-K, Chino M, Mori S (1994) Plant Soil 165:173–179
Noma M, Noguchi M, Tamaki E (1971) Tetrahedron Lett 22:2017–2020
Kristensen I, Larsen PO (1974) Phytochemistry 13:2791–2798
Nomoto K, Mino Y, Ishida T, Yoshioka H, Ota N, Inoue M, Takagi S, Takemoto T (1981) Chem Comm 7:338–339
Stephan UW, Schmidke I, Stephan VW, Scholz G (1996) Biometals 9:84–90
Douchkov D, Herbik A, Koch G, Mock HP, Melzer M, Stephan UW, Baeumlein H (2002) Plant Soil 241:115–119
Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa Naoko K (2003) Plant Cell 15:1263–1280
Scholz G, Schlesier G, Seifert K (1985) Physiol Plant 63:99–104
Budesinsky M, Budzikiewicz H, Prochazka Z, Ripperger H, Roemer A, Scholz G, Schreiber K (1980) Phytochemistry 19:2295–2297
Stephan UW, Scholz G (1993) Physiol Plant 88:522–529
Ling HQ, Pich A, Scholz G, Ganal MW (1996) Mol Gen Genet 252:87–92
Pich A, Scholz G (1996) J Exp Bot 47:41–47
Von Wiren N, Klair S, Bansal S, Briat J-F, Khodr H, Shioiri T, Leigh RA, Hider RC (1999) Plant Physiol 119:1107–1114
Pich A, Manteuffel R, Hillmer S, Scholz G, Schmidt W (2001) Planta 213:967–976
Weber M, Harada E, Vess C, Roepenack-Lahaye von E, Clemens S (2004) Plant J 37:269–281
Rauser WE (1999) Cell Biochem Biophys 31:19–48
Vacchina V, Mari S, Czernic P, Marques L, Pianelli K, Schaumlöffel D, Lebrun M, Lobinski R (2003) Anal Chem 75:2740–2745
Kenny PTM, Nomoto K (1995) J Mass Spectrom:S13–S18
Becher M, Talke IN, Krall L, Krämer U (2004) Plant J 37:251–268
Douchkov D, Gryczka C, Stephan UW, Hell R, Baeumlein H (2005) Plant Cell Environ 28:365–374
Shriver DF, Atkins PW (1999) Inorganic chemistry, 3rd edn. Oxford University Press, Oxford
Matsuura F, Hamada Y, Shioiri T (1994) Tetrahedron 50:9457–9470
Matsuura F, Hamada Y, Shiori T (1994) Tetrahedron 50:265–274
Shioiri T, Irako N, Sakakibara S, Matsuura F, Hamada Y (1997) Heterocycles 44:519–530
Klair SS, Mohan HR, Kitahara T (1998) Tetrahedron Lett 39:89–92
Miyakoshi K, Oshita J, Kitahara T (2001) Tetrahedron 57:3355–3360
Scholz G, Faust J, Ripperger H, Schreiber K (1988) Phytochemistry 27:2749–2754
Anderegg G, Ripperger H (1989) Chem Comm 10:647–650
Hamer DH (1986) Annu Rev Biochem 55:913–951
Martell AE, Hancock RD (1996) Metal complexes in aqueous solutions. Plenum, New York
Calderone V, Dolderer B, Hartmann H-J, Echner H, Luchinat C, Del Bianco C, Mangani S, Weser U (2005) Proc Natl Acad Sci USA 102:51–56
Zhou J, Goldsbrough PB (1995) Mol Gen Genet 248:318–328
Murphy A, Taiz L (1995) Plant Physiol 109:945–954
Cobbett C, Goldsbrough P (2002) Annu Rev Plant Biol 53:159–182
Schat H, Llugany M, Vooijs R, Hartley-Whitaker J, Bleeker Petra M (2002) J Exp Bot 53:2381–2392
Gawel JE, Ahner BA, Friedland AJ, Morel FMM (1996) Nature 381:64–65
Maitani T, Kubota H, Sato K, Yamada T (1996) Plant Physiol 110:1145–1150
Ebbs S, Lau I, Ahner B, Kochian L (2002) Planta 214:635–640
Lee S, Moon JS, Ko T-S, Petros D, Goldsbrough PB, Korban SS (2003) Plant Physiol 131:656–663
Cobbett CS, Goldsbrough PB (2000) In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment, Wiley, New York, pp 247–269
Zhao FJ, Wang JR, Barker JHA, Schat H, Bleeker PM, McGrath SP (2003) New Phytol 159:403–410
Zhang W, Cai Y, Downum KR, Ma LQ (2004) J Chromatogr A 1043:249–254
Zhang W, Cai Y, Downum KR, Ma LQ (2004) Environ Pollut 131:337–345
Raab A, Feldmann J, Meharg AA (2004) Plant Physiol 134:1113–1122
Webb SM, Gaillard J-F, Ma LQ, Tu C (2003) Environ Sci Technol 37:754–760
Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE (2000) Plant Physiol 122:1171–1177
Eitinger T, Mandrand-Berthelot M-A (2000) Arch Microbiol 173:1–9
Eitinger T, Suhr J, Moore L, Smith JAC (2005) Biometals 18:399–405
Baker AJM, Reeves RD, Hajar ASM (1994) New Phytol 127:61–68
Kelly RA, Andrews JC, DeWitt JG (2002) Microchem J 71:231–245
Szpunar J (2005) Analyst 130:442–465
Szpunar J (2000) Analyst 125:963–988
Pence NS, Larsen PB, Ebbs SD, Letham DL, Lasat MM, Garvin DF, Eide D, Kochian LV (2000) Proc Natl Acad Sci USA 97:4956–4960
McGrath SP (1998) In: Brooks RR (ed) Plants that hyperaccumulate heavy metals. CAB Int., Wallingford, UK, pp 261–287
Benes I, Schreiber K, Ripperger H, Kircheiss A (1983) Experientia 39:261–262
Martell AE, Smith RM (1974) Critical stability constants. Plenum, New York
Perrin DD (1979) Stability constants of metal-ion complexes, 2nd edn. Pergamon, Oxford
Herbik A, Koch G, Mock HP, Dushkov D, Czihal A, Thielmann J, Stephan UW, Baumlein H (1999) Eur J Biochem 265:231–239
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Callahan, D.L., Baker, A.J.M., Kolev, S.D. et al. Metal ion ligands in hyperaccumulating plants. J Biol Inorg Chem 11, 2–12 (2006). https://doi.org/10.1007/s00775-005-0056-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-005-0056-7