Abstract
Objectives
The aim was to evaluate the effect of three nitrogen-containing bisphosphonates at different concentrations on osteoblast growth, differentiation, and antigenic profile, using the MG-63 cell line as osteoblast model, in order to determine the role of osteoblasts in bisphosphonate-related osteonecrosis of the jaw (BRONJ).
Materials and methods
Osteoblasts were incubated in culture medium with 10−5, 10−7, or 10−9 M of pamidronate, alendronate, or ibandronate. Proliferative capacity of the osteoblasts was determined by spectrophotometry (MTT) at 24 and 48 h of culture. Flow cytometry was used to study antigenic profile (CD54, CD80, CD86, HLA-DR) and phagocytic activity. Cell differentiation was evaluated at 7, 15, and 21 days by the study of nodule formation and alkaline phosphatase activity (ALP) at 24 h by spectrophotometric assay.
Results
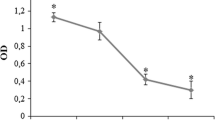
Pamidronate, alendronate, and ibandronate each exerted a significant stimulatory effect on MG63 proliferation that depended on the dose and treatment duration (p < 0.05). In general, a significantly decreased expression of CD54, CD80, and HLA-DR membrane antigens was observed after 24 h of treatment with each nitrogen-containing bisphosphonate (p < 0.05), but there was no significant difference in phagocytic activity versus controls. A decrease in ALP activity was observed after 24 h of treatment and a decrease in calcium deposition after 15 and 21 days (p < 0.05).
Conclusion
Nitrogen-containing bisphosphonates can increase the proliferation of MG-63 osteoblast-like cells, modulate their expression of co-stimulatory molecules associated with immune function, and decrease their differentiation capacity, generally at low doses.
Clinical relevance
These findings suggest that low doses of nitrogen-containing bisphosphonates exert their effect on osteoblasts by altering their physiology, which would explain the disruption of their repair capacity and may be directly related to the development of BRONJ.





Similar content being viewed by others
References
Eggelmeijer F, Papapoulos SE, van Paassen HC et al (1994) Clinical and biochemical response to single infusion of pamidronate in patients with active rheumatoid arthritis: a double blind placebo controlled study. J Rheumatol 21:2016–2020
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Lane JM, Khan SN, O’Connor WJ et al (2001) Bisphosphonate therapy in fibrous dysplasia. Clin Orthop 6–12
Fleisch H (2002) Development of bisphosphonates. Breast Cancer Res BCR 4:30–34
Frith JC, Mönkkönen J, Auriola S et al (2001) The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum 44:2201–2210
Russell RGG (2007) Bisphosphonates: mode of action and pharmacology. Pediatrics 119(Suppl 2):S150–S162
Silverman SL, Maricic M (2007) Recent developments in bisphosphonate therapy. Semin Arthritis Rheum 37:1–12
Mashiba T, Mori S, Burr DB et al (2005) The effects of suppressed bone remodeling by bisphosphonates on microdamage accumulation and degree of mineralization in the cortical bone of dog rib. J Bone Miner Metab 23(Suppl):36–42
Santini D, Vincenzi B, Avvisati G et al (2002) Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin Cancer Res 8:1080–1084
Landesberg R, Cozin M, Cremers S et al (2008) Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg 66:839–847
Marx RE (2003) Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 61:1115–1117
Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL (2004) Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62:527–534
Neve A, Corrado A, Cantatore FP (2011) Osteoblast physiology in normal and pathological conditions. Cell Tissue Res 343:289–302
Eriksen EF (2010) Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord 11:219–227
Ruiz C, Pérez E, Vallecillo-Capilla M, Reyes-Botella C (2003) Phagocytosis and allogeneic T cell stimulation by cultured human osteoblast-like cells. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 13:309–314
Ruiz C, Pérez E, García-Martínez O et al (2007) Expression of cytokines IL-4, IL-12, IL-15, IL-18, and IFNgamma and modulation by different growth factors in cultured human osteoblast-like cells. J Bone Miner Metab 25:286–292
Reyes-Botella C, Montes MJ, Vallecillo-Capilla MF et al (2002) Antigenic phenotype of cultured human osteoblast-like cells. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 12:359–364
Reyes-Botella C, Montes MJ, Vallecillo-Capilla MF et al (2000) Expression of molecules involved in antigen presentation and T cell activation (HLA-DR, CD80, CD86, CD44 and CD54) by cultured human osteoblasts. J Periodontol 71:614–617
Pérez E, García-Martínez O, Arroyo-Morales M et al (2006) Modulation of antigenic phenotype in cultured human osteoblast-like cells by FGFb, TGFbeta1, PDGF-BB, IL-2, IL-1beta, LPS and IFNgamma. Biosci Rep 26:281–289
Lisignoli G, Toneguzzi S, Piacentini A et al (2004) Recruitment and proliferation of T lymphocytes is supported by IFNgamma- and TNFalpha-activated human osteoblasts: Involvement of CD54 (ICAM-1) and CD106 (VCAM-1) adhesion molecules and CXCR3 chemokine receptor. J Cell Physiol 198:388–398
Stanley KT, VanDort C, Motyl C et al (2006) Immunocompetent properties of human osteoblasts: interactions with T lymphocytes. J Bone Miner Res 21:29–36
Díaz-Rodríguez L, García-Martínez O, Arroyo-Morales M et al (2009) Antigenic phenotype and phagocytic capacity of MG-63 osteosarcoma line. Ann N Y Acad Sci 1173(Suppl 1):E46–E54
Rifas L, Arackal S, Weitzmann MN (2003) Inflammatory T cells rapidly induce differentiation of human bone marrow stromal cells into mature osteoblasts. J Cell Biochem 88:650–659
Chen T, Berenson J, Vescio R et al (2002) Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol 42:1228–1236
Manzano-Moreno FJ, Rodríguez-Martínez JB, Ramos-Torrecillas J et al (2013) Proliferation and osteogenic differentiation of osteoblast-like cells obtained from two techniques for harvesting intraoral bone grafts. Clin Oral Investig 17:1349–1356
Sandrini E, Morris C, Chiesa R et al (2005) In vitro assessment of the osteointegrative potential of a novel multiphase anodic spark deposition coating for orthopaedic and dental implants. J Biomed Mater Res B Appl Biomater 73:392–399
Krischak GD, Augat P, Blakytny R et al (2007) The non-steroidal anti-inflammatory drug diclofenac reduces appearance of osteoblasts in bone defect healing in rats. Arch Orthop Trauma Surg 127:453–458
Eingartner C, Coerper S, Fritz J et al (1999) Growth factors in distraction osteogenesis. Immuno-histological pattern of TGF-beta1 and IGF-I in human callus induced by distraction osteogenesis. Int Orthop 23:253–259
Wang FS, Yang KD, Chen RF et al (2002) Extracorporeal shock wave promotes growth and differentiation of bone-marrow stromal cells towards osteoprogenitors associated with induction of TGF-beta1. J Bone Joint Surg (Br) 84:457–461
Chen Y-J, Wurtz T, Wang C-J et al (2004) Recruitment of mesenchymal stem cells and expression of TGF-beta 1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J Orthop Res 22:526–534
Boanini E, Torricelli P, Gazzano M et al (2014) Combined effect of strontium and zoledronate on hydroxyapatite structure and bone cell responses. Biomaterials 35:5619–5626
Mattioli-Belmonte M, Cometa S, Ferretti C, et al. (2014) Characterization and cytocompatibility of an antibiotic/chitosan/cyclodextrins nanocoating on titanium implants. Carbohydr Polym 22;110:173–82
Lee YJ, Jeong JK, Seol JW, et al. (2013) Activated protein C differentially regulates both viability and differentiation of osteoblasts mediated by bisphosphonates. Exp Mol Med 15;45:e9
Im G-I, Qureshi SA, Kenney J et al (2004) Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 25:4105–4115
Xiong Y, Yang HJ, Feng J et al (2009) Effects of alendronate on the proliferation and osteogenic differentiation of MG-63 cells. J Int Med Res 37:407–416
Kim HK, Kim JH, Abbas AA, Yoon TR (2009) Alendronate enhances osteogenic differentiation of bone marrow stromal cells: a preliminary study. Clin Orthop 467:3121–3128
Idris AI, Rojas J, Greig IR et al (2008) Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int 82:191–201
Orriss IR, Key ML, Colston KW, Arnett TR (2009) Inhibition of osteoblast function in vitro by aminobisphosphonates. J Cell Biochem 106:109–118
Frediani B, Spreafico A, Capperucci C et al (2004) Long-term effects of neridronate on human osteoblastic cell cultures. Bone 35:859–869
De Luna-Bertos E, Ramos-Torrecillas J, García-Martínez O et al (2013) Therapeutic doses of nonsteroidal anti-inflammatory drugs inhibit osteosarcoma MG-63 osteoblast-like cells maturation, viability, and biomineralization potential. ScientificWorldJournal 2013:809891
Díaz-Rodríguez L, García-Martínez O, Morales MA et al (2012) Effects of indomethacin, nimesulide, and diclofenac on human MG-63 osteosarcoma cell line. Biol Res Nurs 14:98–107
García-Martínez O, Díaz-Rodríguez L, Rodríguez-Pérez L et al (2011) Effect of acetaminophen, ibuprofen and methylprednisolone on different parameters of human osteoblast-like cells. Arch Oral Biol 56:317–323
Ing SW, Belury MA (2011) Impact of conjugated linoleic acid on bone physiology: proposed mechanism involving inhibition of adipogenesis. Nutr Rev 69:123–131
Naidu A, Dechow PC, Spears R et al (2008) The effects of bisphosphonates on osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:5–13
Gebken J, Feydt A, Brinckmann J et al (1999) Ligand-induced downregulation of receptors for TGF-beta in human osteoblast-like cells from adult donors. J Endocrinol 161:503–510
Balooch G, Balooch M, Nalla RK et al (2005) TGF-beta regulates the mechanical properties and composition of bone matrix. Proc Natl Acad Sci U S A 102:18813–18818
Walter C, Al-Nawas B, Frickhofen N et al (2010) Prevalence of bisphosphonate associated osteonecrosis of the jaws in multiple myeloma patients. Head Face Med 6:11
Boonyapakorn T, Schirmer I, Reichart PA et al (2008) Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol 44:857–869
Yépez Guillén JV, Martínez de Páez N (2009) Gottberg de Nogueira E osteonecrosis de los maxilares inducida por bifosfonatos. Rev Odontol Andes 4:43–54
Marx RE, Cillo JE Jr, Ulloa JJ (2007) Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg 65:2397–2410
Walter C, Grötz KA, Kunkel M, Al-Nawas B (2007) Prevalence of bisphosphonate associated osteonecrosis of the jaw within the field of osteonecrosis. Support Care Cancer 15:197–202
Nancollas GH, Tang R, Phipps RJ et al (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627
Acknowledgments
This study was supported by research group BIO277 (Junta de Andalucía).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manzano-Moreno, F.J., Ramos-Torrecillas, J., De Luna-Bertos, E. et al. Nitrogen-containing bisphosphonates modulate the antigenic profile and inhibit the maturation and biomineralization potential of osteoblast-like cells. Clin Oral Invest 19, 895–902 (2015). https://doi.org/10.1007/s00784-014-1309-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1309-z