Abstract
Objective
The aim if this study was to determine the minimal inhibitory concentrations of chlorhexidine digluconate and an amine fluoride/stannous fluoride-containing mouthrinse against Porphyromonas gingivalis and mutans streptococci during an experimental long-term subinhibitory exposition.
Material and methods
Five P. gingivalis strains and four mutans streptococci were subcultivated for 20–30 passages in subinhibitory concentrations of chlorhexidine digluconate or an amine fluoride/stannous fluoride-containing mouthrinse.
Results
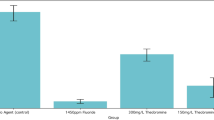
Pre-passaging minimal inhibitory concentrations for chlorhexidine ranged from 0.5 to 2 mg/l for mutans streptococci and from 2 to 4 mg/l for the P. gingivalis isolates. For the amine fluoride/stannous fluoride-containing mouthrinse minimal inhibitory values from 0.125 to 0.25 % for the mutans streptococci and from 0.063 to 0.125 % for the P. gingivalis isolates were determined. Two- to fourfold increased minimal inhibitory concentrations against chlorhexidine were detected for two of the five P. gingivalis isolates, whereas no increase in minimal inhibitory concentrations was found for the mutans streptococci after repeated passaging through subinhibitory concentrations. Repeated exposure to subinhibitory concentrations of the amine fluoride/stannous fluoride-containing mouthrinse did not alter the minimally inhibitory concentrations of the bacterial isolates tested.
Conclusion
Chlorhexidine and the amine fluoride/stannous fluoride-containing mouthrinse are effective inhibitory agents against the oral bacterial isolates tested. No general development of resistance against chlorhexidine or the amine fluoride/stannous fluoride-containing mouthrinse was detected. However, some strains showed potential to develop resistance against chlorhexidine after prolonged exposure.
Clinical relevance
The use of chlorhexidine should be limited to short periods of time. The amine fluoride/stannous fluoride-containing mouthrinse appears to have the potential to be used on a long-term basis.




Similar content being viewed by others
References
Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE (2005) Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43:5721–5732
Albandar JM, Tinoco EM (2002) Global epidemiology of periodontal diseases in children and young persons. Periodontol 2000 29:153–176
Bostanci N, Belibasakis GN (2012) Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett 333:1–9
Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F (2011) Resistance of bacterial biofilms to disinfectants: a review. Biofouling 27:1017–1032
Clinical and Laboratory Standards Institute (2007) Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard M11-A7. CLSI, Wayne, USA
Davies A, Roberts W (1969) The cell wall of a chlorhexidine-resistant Pseudomonas. Biochem J 112:15P
Davies GE, Francis J, Martin AR, Rose FL, Swain G (1954) 1:6-Di-4'-chlorophenyldiguanidohexane (hibitane); laboratory investigation of a new antibacterial agent of high potency. Br J Pharmacol Chemother 9:192–196
de Lastours V, Cambau E, Guillard T, Marcade G, Chau F, Fantin B (2012) Diversity of individual dynamic patterns of emergence of resistance to quinolones in Escherichia coli from the fecal flora of healthy volunteers exposed to ciprofloxacin. J Infect Dis 206:1399–1406
De Soete M, Dekeyser C, Pauwels M, Teughels W, van Steenberghe D, Quirynen M (2005) Increase in cariogenic bacteria after initial periodontal therapy. J Dent Res 84:48–53
Do LG (2012) Distribution of caries in children: variations between and within populations. J Dent Res 91:536–543
Eick S, Rodel J, Einax JW, Pfister W (2002) Interaction of Porphyromonas gingivalis with KB cells: comparison of different clinical isolates. Oral Microbiol Immunol 17:201–208
Eick S, Goltz S, Nietzsche S, Jentsch H, Pfister W (2011) Efficacy of chlorhexidine digluconate-containing formulations and other mouthrinses against periodontopathogenic microorganisms. Quintessence Int 42:687–700
el Moug T, Rogers DT, Furr JR, el-Falaha BM, Russell AD (1985) Antiseptic-induced changes in the cell surface of a chlorhexidine-sensitive and a chlorhexidine-resistant strain of Providencia stuartii. J Antimicrob Chemother 16:685–689
Fang CT, Chen HC, Chuang YP, Chang SC, Wang JT (2002) Cloning of a cation efflux pump gene associated with chlorhexidine resistance in Klebsiella pneumoniae. Antimicrob Agents Chemother 46:2024–2028
Flisfisch S, Meyer J, Meurman JH, Waltimo T (2008) Effects of fluorides on Candida albicans. Oral Dis 14:296–301
Flötra L, Gjermo P, Rolla G, Waerhaug J (1971) Side effects of chlorhexidine mouth washes. Scand J Dent Res 79:119–125
Gnanadhas DP, Marathe SA, Chakravortty D (2013) Biocides–resistance, cross-resistance mechanisms and assessment. Expert Opin Investig Drugs 22:191–206
Guggenheim B, Schroeder HE (1967) Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta 11:131–152
Hamada S, Slade HD (1980) Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev 44:331–384
Jarvinen H, Tenovuo J, Huovinen P (1993) In vitro susceptibility of Streptococcus mutans to chlorhexidine and six other antimicrobial agents. Antimicrob Agents Chemother 37:1158–1159
Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A (2013) Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55:93–100
Kosutic D, Uglesic V, Perkovic D, Persic Z, Solman L, Lupi-Ferandin S, Knezevic P, Sokler K, Knezevic G (2009) Preoperative antiseptics in clean/contaminated maxillofacial and oral surgery: prospective randomized study. Int J Oral Maxillofac Surg 38:160–165
Kulik E, Lenkeit K, Meyer J (2000) Antimicrobial effects of tea tree oil (Melaleuca alternifolia) on oral microorganisms. Schweiz Monatsschr Zahnmed 110:125–130
Kulik EM, Lenkeit K, Chenaux S, Meyer J (2008) Antimicrobial susceptibility of periodontopathogenic bacteria. J Antimicrob Chemother 61:1087–1091
Kulik Kunz EM, Lenkeit K, Waltimo T, Weiger R, Walter C (2004) Combinatorial effects of amoxicillin and metronidazole on selected periodontal bacteria and whole plaque samples. Arch Oral Biol 59:608–615
Meurman JH, Jousimies-Somer H, Suomala P, Alaluusua S, Torkko H, Asikainen S (1989) Activity of amine-stannous fluoride combination and chlorhexidine against some aerobic and anaerobic oral bacteria. Oral Microbiol Immunol 4:117–119
Micheelis W (2011) Oral health in Germany: an oral epidemiological outline. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 54:1022–1026
Moore WE, Moore LV (1994) The bacteria of periodontal diseases. Periodontol 2000 5:66–77
Morrissey I, Oggioni MR, Knight D, Curiao T, Coque T, Kalkanci A, Martinez JL (2014) Evaluation of epidemiological cut-off values indicates that biocide resistant subpopulations are uncommon in natural isolates of clinically-relevant microorganisms. PLoS One 9:e86669
Nagai I, Ogase H (1990) Absence of role for plasmids in resistance to multiple disinfectants in three strains of bacteria. J Hosp Infect 15:149–155
Nakahara H, Kozukue H (1981) Chlorhexidine resistance in Escherichia coli isolated from clinical lesions. Zentralbl Bakteriol Mikrobiol Hyg A 251:177–184
Nakahara H, Kozukue H (1982) Isolation of chlorhexidine-resistant Pseudomonas aeruginosa from clinical lesions. J Clin Microbiol 15:166–168
Netuschil L, Weiger R, Preisler R, Brecx M (1995) Plaque bacteria counts and vitality during chlorhexidine, Meridol and Listerine mouthrinses. Eur J Oral Sci 103:355–361
Nicoletti G, Boghossian V, Gurevitch F, Borland R, Morgenroth P (1993) The antimicrobial activity in vitro of chlorhexidine, a mixture of isothiazolinones ('Kathon' CG) and cetyl trimethyl ammonium bromide (CTAB). J Hosp Infect 23:87–111
Quirynen M, Mongardini C, Pauwels M, Bollen CM, Van Eldere J, van Steenberghe D (1999) One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. II. Long-term impact on microbial load. J Periodontol 70:646–656
Rohrer N, Widmer AF, Waltimo T, Kulik EM, Weiger R, Filipuzzi-Jenny E, Walter C (2010) Antimicrobial efficacy of 3 oral antiseptics containing octenidine, polyhexamethylene biguanide, or Citroxx: can chlorhexidine be replaced? Infect Control Hosp Epidemiol 31:733–739
Schürch E Jr, Lang NP (2004) Periodontal conditions in Switzerland at the end of the 20th century. Oral Health Prev Dent 2:359–368
Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr (1998) Microbial complexes in subgingival plaque. J Clin Periodontol 25:134–144
Solmaz G, Korachi M (2012) Inhibition and disruption properties of chlorhexidine gluconate on single and multispecies oral biofilms. Jundishapur J Microbiol 6:61–66
Stickler DJ (1974) Chlorhexidine resistance in Proteus mirabilis. J Clin Pathol 27:284–287
Stickler DJ (2002) Susceptibility of antibiotic-resistant Gram-negative bacteria to biocides: a perspective from the study of catheter biofilms. J Appl Microbiol 92:163S–170S
Tambe SM, Sampath L, Modak SM (2001) In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J Antimicrob Chemother 47:589–598
Tumah HN (2009) Bacterial biocide resistance. J Chemother 21:5–15
van Winkelhoff AJ, Herrera D, Oteo A, Sanz M (2005) Antimicrobial profiles of periodontal pathogens isolated from periodontitis patients in The Netherlands and Spain. J Clin Periodontol 32:893–898
Walter C, Zahlten J, Schmeck B, Schaudinn C, Hippenstiel S, Frisch E, Hocke AC, Pischon N, Kuramitsu HK, Bernimoulin JP, Suttorp N, Krull M (2004) Porphyromonas gingivalis strain-dependent activation of human endothelial cells. Infect Immun 72:5910–5918
Walter C, Purucker P, Bernimoulin JP, Suttorp N, Meyer J, Weiger R (2005) Critical assessment of microbiological diagnostics in periodontal diseases with special focus on Porphyromonas gingivalis. Schweiz Monatsschr Zahnmed 115:415–424
Walter C, Weiger R (2006) Antibiotics as the only therapy of untreated chronic periodontitis: a critical commentary. J Clin Periodontol 33:938–939
Walter C, Buset S, Thillainathan L, Weiger R, Zitzmann NU (2013) Evaluation of periodontal therapy in undergraduate courses of the University of Basle. A retrospective study. Schweiz Monatsschr Zahnmed 123:861–877
Westbrook SD, Wiederhold NP, Vallor AC, Kotara S, Bernardo S, Lee SA, Kirkpatrick WR, Toro JJ, Freytes C, Patterson TF, Redding SW (2010) Loss of in vitro resistance in Candida glabrata following discontinuation of fluconazole prophylaxis in a hematopoietic stem cell transplantation patient. Med Mycol 48:557–560
Zahlten J, Riep B, Nichols FC, Walter C, Schmeck B, Bernimoulin JP, Hippenstiel S (2007) Porphyromonas gingivalis dihydroceramides induce apoptosis in endothelial cells. J Dent Res 86:635–640
Zhang TT, Tang SS, Fu LJ (2013) The effectiveness of different concentrations of chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. J Clin Nurs 23:1461–1475
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kulik, E.M., Waltimo, T., Weiger, R. et al. Development of resistance of mutans streptococci and Porphyromonas gingivalis to chlorhexidine digluconate and amine fluoride/stannous fluoride-containing mouthrinses, in vitro. Clin Oral Invest 19, 1547–1553 (2015). https://doi.org/10.1007/s00784-014-1379-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1379-y