Abstract
Objectives
Alveolar bone structures are mostly investigated in small animal models. The majority of these studies examined local influences on the alveolar bone, but only a few examined systemic influencing factors. The hypothalamic-pituitary axis is known to be essential for a vital bone balance. The aim of this study was to analyse the effects that selective hormone treatments have on alveolar bone structure and quality in a sheep model for alveolar bone loss, induced by hypothalamic-pituitary disconnection (HPD).
Methods
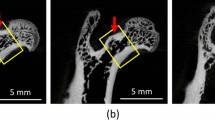
Thirty sheep were randomly selected into six groups of five each: control (C), ovariectomy—OVX (O), O + HPD (OH), OH with oestrogen treatment (OHE), OH with thyroxine (T4) treatment (OHT), and OH with a combined treatment of oestrogen and thyroxine (OHTE). After OVX and HPD procedures and an additional 9-month observation/treatment period, structural bone analyses of the mandible were performed by contact radiography, micro-CT, and static histomorphometry.
Results
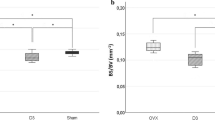
The HPD procedure caused structural alveolar bone parameters to decrease significantly compared to controls (C). Treatment with oestrogen (OHE) was protective and bone structure was maintained at baseline levels. Thyroxine treatment (OHT) promoted significant bone loss, but the combined treatment (OHTE) improved bone structure and volume parameters even above baseline levels.
Conclusions
Alveolar bone homeostasis significantly underlies systemic regulatory systems. Centrally induced (HPD) bone loss can be prevented by combined peripheral treatment with oestrogen and thyroxine.
Clinical relevance
These results demonstrate the significance of a balanced hormonal regulatory system for steady bone remodelling and maintenance of healthy alveolar bone.




Similar content being viewed by others
References
Rawlinson SC (2009) Ovariectomy vs. hypofunction: their effects on rat mandibular bone. J Dent Res 88:615–620. doi:10.1177/0022034509340132
Sanfilippo F, Bianchi AE (2003) Osteoporosis: the effect on maxillary bone resorption and therapeutic possibilities by means of implant prostheses—a literature review and clinical considerations. Int J Periodontics Restorative Dent 23:447–457
Tatakis DN, Kumar PS (2005) Etiology and pathogenesis of periodontal diseases. Dent Clin North Am 49:491-516, v. doi:10.1016/j.cden.2005.03.001
Kribbs PJ, Chesnut CH 3rd, Ott SM, Kilcoyne RF (1989) Relationships between mandibular and skeletal bone in an osteoporotic population. J Prosthet Dent 62:703–707
Krall EA, Garcia RI, Dawson-Hughes B (1996) Increased risk of tooth loss is related to bone loss at the whole body, hip, and spine. Calcif Tissue Int 59:433–437
Jonasson G, Jonasson L, Kiliaridis S (2006) Changes in the radiographic characteristics of the mandibular alveolar process in dentate women with varying bone mineral density: a 5-year prospective study. Bone 38:714–721. doi:10.1016/j.bone.2005.10.008
Oz HS, Puleo DA (2011) Animal models for periodontal disease. J Biomed Biotechnol 2011:754857. doi:10.1155/2011/754857
Yamashiro T, Takano-Yamamoto T (2001) Influences of ovariectomy on experimental tooth movement in the rat. J Dent Res 80:1858–1861
Tanaka M, Ejiri S, Toyooka E, Kohno S, Ozawa H (2002) Effects of ovariectomy on trabecular structures of rat alveolar bone. J Periodontal Res 37:161–165
Tanaka M, Toyooka E, Kohno S, Ozawa H, Ejiri S (2003) Long-term changes in trabecular structure of aged rat alveolar bone after ovariectomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95:495–502. doi:10.1067/moe.2003.135
Rawlinson SC, Boyde A, Davis GR, Howell PG, Hughes FJ, Kingsmill VJ (2009) Ovariectomy vs. hypofunction: their effects on rat mandibular bone. J Dent Res 88:615–620. doi:10.1177/0022034509340132
Dvorak G, Gruber R, Huber CD, Goldhahn J, Zanoni G, Salaberger D, Watzek G, Haas R (2008) Trabecular bone structures in the edentulous diastema of osteoporotic sheep. J Dent Res 87:866–870
Dvorak G, Reich KM, Tangl S, Goldhahn J, Haas R, Gruber R (2011) Cortical porosity of the mandible in an osteoporotic sheep model. Clin Oral Implants Res 22:500–505. doi:10.1111/j.1600-0501.2010.02031.x
Beil FT, Oheim R, Barvencik F, Hissnauer TN, Pestka JM, Ignatius A, Rueger JM, Schinke T, Clarke IJ, Amling M, Pogoda P (2012) Low turnover osteoporosis in sheep induced by hypothalamic-pituitary disconnection. J Orthop Res 30:1254–1262. doi:10.1002/jor.22066
Oheim R, Beil FT, Krause M, Bindl R, Ignatius A, Pogoda P (2013) Mandibular bone loss in ewe induced by hypothalamic-pituitary disconnection. Clin Oral Implants Res 25:1239–1244. doi:10.1111/clr.12259
Oheim R, Beil FT, Kohne T, Wehner T, Barvencik F, Ignatius A, Amling M, Clarke IJ, Pogoda P (2013) Sheep model for osteoporosis: sustainability and biomechanical relevance of low turnover osteoporosis induced by hypothalamic-pituitary disconnection. J Orthop Res 31:1067–1074. doi:10.1002/jor.22327
Oheim R, Schinke T, Amling M, Pogoda P (2016) Can we induce osteoporosis in animals comparable to the human situation? Injury 47(Suppl 1):S3–S9. doi:10.1016/S0020-1383(16)30002-X
Clarke IJ, Cummins JT, de Kretser DM (1983) Pituitary gland function after disconnection from direct hypothalamic influences in the sheep. Neuroendocrinology 36:376–384
Prickett TC, Barrell GK, Wellby M, Yandle TG, Richards AM, Espiner EA (2008) Effect of sex steroids on plasma C-type natriuretic peptide forms: stimulation by oestradiol in lambs and adult sheep. J Endocrinol 199:481–487. doi:10.1677/JOE-08-0267
Billings HJ, Viguie C, Karsch FJ, Goodman RL, Connors JM, Anderson GM (2002) Temporal requirements of thyroid hormones for seasonal changes in LH secretion. Endocrinology 143:2618–2625
Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB (1999) Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 140:4982–4987
Oheim R, Amling M, Ignatius A, Pogoda P (2012) Large animal model for osteoporosis in humans: the ewe. Eur Cell Mater 24:372–385
Pogoda P, Egermann M, Schnell JC, Priemel M, Schilling AF, Alini M, Schinke T, Rueger JM, Schneider E, Clarke I, Amling M (2006) Leptin inhibits bone formation not only in rodents, but also in sheep. J Bone Miner Res 21:1591–1599. doi:10.1359/jbmr.060709
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee J Bone Miner Res 2:595–610. doi:10.1002/jbmr.5650020617
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28:2–17. doi:10.1002/jbmr.1805
Oheim R, Simon MJ, Steiner M, Vettorazzi E, Barvencik F, Ignatius A, Amling M, Clarke IJ, Pogoda P, Beil FT (2017) Sheep model for osteoporosis: the effects of peripheral hormone therapy on centrally induced systemic bone loss in an osteoporotic sheep model. Injury. doi:10.1016/j.injury.2017.02.009
Turner AS, Mallinckrodt CH, Alvis MR, Bryant HU (1995) Dose-response effects of estradiol implants on bone mineral density in ovariectomized ewes. Bone 17:421S–427S
Duncan WJ, Lee MH, Bae TS, Lee SJ, Gay J, Loch C (2015) Anodisation increases integration of unloaded titanium implants in sheep mandible. Biomed Res Int 2015:857969. doi:10.1155/2015/857969
Trisi P, Berardini M, Falco A, Podaliri Vulpiani M, Perfetti G (2014) Insufficient irrigation induces peri-implant bone resorption: an in vivo histologic analysis in sheep. Clin Oral Implants Res 25:696–701. doi:10.1111/clr.12127
Driessler F, Baldock PA (2010) Hypothalamic regulation of bone. J Mol Endocrinol 45:175–181. doi:10.1677/JME-10-0015
Raisz LG (1999) Physiology and pathophysiology of bone remodeling. Clin Chem 45:1353–1358
Pacifici R (1998) Cytokines, estrogen, and postmenopausal osteoporosis—the second decade. Endocrinology 139:2659–2661. doi:10.1210/endo.139.6.6087
Khosla S, Oursler MJ, Monroe DG (2012) Estrogen and the skeleton. Trends Endocrinol Metab 23:576–581. doi:10.1016/j.tem.2012.03.008
De Rosa G, Testa A, Giacomini D, Carrozza C, Astazi P, Caradonna P (1997) Prospective study of bone loss in pre- and post-menopausal women on L-thyroxine therapy for non-toxic goitre. Clin Endocrinol 47:529–535
Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, Iqbal J, Eldeiry L, Rajendren G, Blair HC, Davies TF, Zaidi M (2003) TSH is a negative regulator of skeletal remodeling. Cell 115:151–162
Nicholls JJ, Brassill MJ, Williams GR, Bassett JH (2012) The skeletal consequences of thyrotoxicosis. J Endocrinol 213:209–221. doi:10.1530/JOE-12-0059
Monfoulet LE, Rabier B, Dacquin R, Anginot A, Photsavang J, Jurdic P, Vico L, Malaval L, Chassande O (2011) Thyroid hormone receptor beta mediates thyroid hormone effects on bone remodeling and bone mass. J Bone Miner Res 26:2036–2044. doi:10.1002/jbmr.432
Heino TJ, Hentunen TA, Vaananen HK (2002) Osteocytes inhibit osteoclastic bone resorption through transforming growth factor-beta: enhancement by estrogen. J Cell Biochem 85:185–197
Acknowledgements
The authors appreciate the tremendous help and technical assistance of Mr. Bruce Doughton and Ms. Lynda Morrish and would like to thank them, as well as Ms. Marion Dietzmann, for her help in histological processing. This work was supported by the German Research Foundation (DFG) within the framework of the DFG Research Group 793 to PP and MA. FTB, MJKS, and RO are fellows of the German Research Foundation (DFG AM 103/13-1 and DFG AM 103/14-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the German Research Foundation (DFG) within the framework of the DFG Research Group 793 to PP and MA. FTB, MJKS, and RO are fellows of the German Research Foundation (DFG AM 103/13-1 and DFG AM 103/14-1).
Ethical approval
All applicable national and institutional guidelines for the care and use of animals were followed. All procedures and tissue collections were in accordance with the guidelines for the Care and Maintenance of Experimental Animals and with the prior approval of the Monash Medical Centre Animal Ethics Committee and the Animal Experimentation Ethics Committee of Victorian Institute of Animal Science, Victoria, Australia.
Rights and permissions
About this article
Cite this article
Simon, M.J.K., Beil, F.T., Pogoda, P. et al. Is centrally induced alveolar bone loss in a large animal model preventable by peripheral hormone substitution?. Clin Oral Invest 22, 495–503 (2018). https://doi.org/10.1007/s00784-017-2138-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2138-7