Abstract
Objectives
The objectives of this study were to analyze the effect of pH on the growth and activity of osteoclasts treated with different doses of two nitrogen-containing BPs, zoledronate and alendronate.
Materials and Methods
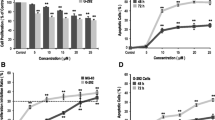
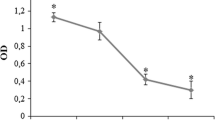
Murine osteoclasts cultured on dentine disks were treated with zoledronate (50 or 500 nM) or alendronate (500 or 5 μM) at two different pH values (7.4 or 7.0). Osteoclasts were counted with transmitted light microscopy, apoptosis/necrosis was studied with flow cytometry and confocal microscopy, and resorption pit number and depth were calculated using reflected light and scanning electron microscopy.
Results
The osteoclast count on dentine disks was significantly (p < 0.001) reduced by zoledronate or alendronate treatment at pH 7.0 in comparison to treatment with the same doses at pH 7.4 and untreated disks (controls). The percentage of apoptotic cells was significantly increased by treatment with 500 nM zoledronate or 5 μM alendronate at pH 7.0 in comparison to the same doses at pH 7.4. The number and depth of resorption pits were significantly lower in disks treated at each BP dose studied than in untreated controls at pH 7.0.
Conclusions
Zoledronate and alendronate at therapeutic doses have an adverse effect on the viability and resorptive activity of osteoclasts when the local medium pH is reduced.
Clinical relevance
These findings suggest that periodontal or peri-implant oral cavity infection may be a key trigger of the cascade of events that lead to BRONJ.





Similar content being viewed by others
References
Fleisch H (1998) Bisphosphonates: mechanisms of action. Endocr Rev 19:80–100. https://doi.org/10.1210/edrv.19.1.0325
Marx RE (2003) Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 61:1115–1117
Marx RE (2014) A decade of bisphosphonate bone complications: what it has taught us about bone physiology. Int J Oral Maxillofac Implants 29:e247–e258
Frith JC, Mönkkönen J, Auriola S et al (2001) The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum 44:2201–2210
Marx RE, Cillo JE Jr, Ulloa JJ (2007) Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg 65:2397–2410. https://doi.org/10.1016/j.joms.2007.08.003
Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B (2009) American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg 67:2–12. https://doi.org/10.1016/j.joms.2009.01.009
Mashiba T, Mori S, Burr DB et al (2005) The effects of suppressed bone remodeling by bisphosphonates on microdamage accumulation and degree of mineralization in the cortical bone of dog rib. J Bone Miner Metab 23(Suppl):36–42
Landesberg R, Cozin M, Cremers S, Woo V, Kousteni S, Sinha S, Garrett-Sinha LA, Raghavan S (2008) Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg 66:839–847. https://doi.org/10.1016/j.joms.2008.01.026
Manzano-Moreno FJ, Ramos-Torrecillas J, De Luna-Bertos E et al (2015) High doses of bisphosphonates reduce osteoblast-like cell proliferation by arresting the cell cycle and inducing apoptosis. J Craniomaxillofac Surg 43:396–401. https://doi.org/10.1016/j.jcms.2014.12.008
Manzano-Moreno FJ, Ramos-Torrecillas J, De Luna-Bertos E et al (2015) Nitrogen-containing bisphosphonates modulate the antigenic profile and inhibit the maturation and biomineralization potential of osteoblast-like cells. Clin Oral Investig 19:895–902. https://doi.org/10.1007/s00784-014-1309-z
Arnett TR (2010) Acidosis, hypoxia and bone. Arch Biochem Biophys 503:103–109. https://doi.org/10.1016/j.abb.2010.07.021
Arnett TR, Dempster DW (1986) Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology 119:119–124. https://doi.org/10.1210/endo-119-1-119
Arnett TR, Dempster DW (1987) A comparative study of disaggregated chick and rat osteoclasts in vitro: effects of calcitonin and prostaglandins. Endocrinology 120:602–608. https://doi.org/10.1210/endo-120-2-602
Arnett TR (2008) Extracellular pH regulates bone cell function. J Nutr 138:415S–418S
Otto S, Schreyer C, Hafner S, Mast G, Ehrenfeld M, Stürzenbaum S, Pautke C (2012) Bisphosphonate-related osteonecrosis of the jaws—characteristics, risk factors, clinical features, localization and impact on oncological treatment. J Craniomaxillofac Surg 40:303–309. https://doi.org/10.1016/j.jcms.2011.05.003
Russell RGG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19:733–759. https://doi.org/10.1007/s00198-007-0540-8
Otto S, Hafner S, Mast G, Tischer T, Volkmer E, Schieker M, Stürzenbaum SR, von Tresckow E, Kolk A, Ehrenfeld M, Pautke C (2010) Bisphosphonate-related osteonecrosis of the jaw: is pH the missing part in the pathogenesis puzzle? J Oral Maxillofac Surg 68:1158–1161. https://doi.org/10.1016/j.joms.2009.07.079
Orriss IR, Arnett TR (2012) Rodent osteoclast cultures. Methods Mol Biol (Clifton NJ) 816:103–117. https://doi.org/10.1007/978-1-61779-415-5_8
De Luna-Bertos E, Ramos-Torrecillas J, Manzano-Moreno FJ et al (2014) Effects on growth of human osteoblast-like cells of three nonsteroidal anti-inflammatory drugs metamizole, dexketoprofen, and ketorolac. Biol Res Nurs 17:62–67. https://doi.org/10.1177/1099800414527155
Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F, Schran H, Seaman J, Skerjanec A (2002) Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol 42:1228–1236
Arnett TR, Spowage M (1996) Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone 18:277–279
Morrison MS, Turin L, King BF, Burnstock G, Arnett TR (1998) ATP is a potent stimulator of the activation and formation of rodent osteoclasts. J Physiol 511(Pt 2):495–500
Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA (1991) Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 88:2095–2105. https://doi.org/10.1172/JCI115539
Hays RC, Mandell GL (1974) PO2, pH, and redox potential of experimental abscesses. Proc Soc Exp Biol Med 147:29–30
Bertram P, Treutner KH, Klosterhalfen B, Arlt G, Anurov M, Polivoda M, Ottinger A, Schumpelick V (1997) Artificial pressure increase in subcutaneous abscess with evidence of general systemic reaction. Langenbecks Arch Chir 382:291–294
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RGG, Ebetino FH (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627. https://doi.org/10.1016/j.bone.2005.05.003
Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC (2000) Cellular and molecular mechanisms of action of bisphosphonates. Cancer 88:2961–2978
Broxmeyer HE, Cooper S, Lu L, Miller ME, Langefeld CD, Ralph P (1990) Enhanced stimulation of human bone marrow macrophage colony formation in vitro by recombinant human macrophage colony-stimulating factor in agarose medium and at low oxygen tension. Blood 76:323–329
Koller MR, Bender JG, Miller WM, Papoutsakis ET (1992) Reduced oxygen tension increases hematopoiesis in long-term culture of human stem and progenitor cells from cord blood and bone marrow. Exp Hematol 20:264–270
Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, Meghji S (2003) Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol 196:2–8. https://doi.org/10.1002/jcp.10321
Lewis JS, Lee JA, Underwood JC et al (1999) Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol 66:889–900
Otto S, Pautke C, Opelz C, Westphal I, Drosse I, Schwager J, Bauss F, Ehrenfeld M, Schieker M (2010) Osteonecrosis of the jaw: effect of bisphosphonate type, local concentration, and acidic milieu on the pathomechanism. J Oral Maxillofac Surg 68:2837–2845. https://doi.org/10.1016/j.joms.2010.07.017
Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL (2004) Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62:527–534
Montazeri AH, Erskine JG, McQuaker IG (2007) Oral sodium clodronate induced osteonecrosis of the jaw in a patient with myeloma. Eur J Haematol 79:69–71. https://doi.org/10.1111/j.1600-0609.2007.00872.x
Ohe J-Y, Kwon Y-D, Lee H-W (2012) Bisphosphonates modulate the expression of OPG and M-CSF in hMSC-derived osteoblasts. Clin Oral Investig 16:1153–1159. https://doi.org/10.1007/s00784-011-0614-z
Sharma A, Raman A, Pradeep AR (2017) Role of 1% alendronate gel as adjunct to mechanical therapy in the treatment of chronic periodontitis among smokers. J Appl Oral Sci Rev FOB 25:243–249. https://doi.org/10.1590/1678-7757-2016-0201
Dutra BC, Oliveira AMSD, Oliveira PAD et al (2017) Effect of 1% sodium alendronate in the non-surgical treatment of periodontal intraosseous defects: a 6-month clinical trial. J Appl Oral Sci Rev FOB 25:310–317. https://doi.org/10.1590/1678-7757-2016-0252
Manzano-Moreno FJ, Ramos-Torrecillas J, Melguizo-Rodríguez L, Illescas-Montes R, Ruiz C, García-Martínez O (2018) Bisphosphonate modulation of the gene expression of different markers involved in osteoblast physiology: possible implications in bisphosphonate-related osteonecrosis of the jaw. Int J Med Sci 15:359–367. https://doi.org/10.7150/ijms.22627
Acknowledgments
We thank Mark Turmaine for assistance with scanning electron micrographs.
Funding
This study was supported by research group BIO277 (Junta de Andalucía).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving animals were in accordance with the ethical standards of the ethical committee of the University of Granada.
Rights and permissions
About this article
Cite this article
Manzano-Moreno, F.J., Ramos-Torrecillas, J., de Luna-Bertos, E. et al. Influence of pH on osteoclasts treated with zoledronate and alendronate. Clin Oral Invest 23, 813–820 (2019). https://doi.org/10.1007/s00784-018-2505-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2505-z