Abstract
Objective
The aim of this study was to evaluate the bone-regeneration efficiency of novel polymeric nanostructured membranes and the effect of zinc, calcium, titanium, and bone morpho-protein loading on membranes, through an in vivo rabbit model.
Material and methods
Nanostructured membranes of methylmethacrylate were loaded with zinc, calcium, TiO2 nanoparticles, and bone-morphogenetic protein (BMP). These membranes covered the bone defects prepared on the skulls of six rabbits. Animals were sacrificed 6 weeks after surgery. Micro computed tomography was used to evaluate bone architecture through BoneJ pluging and ImageJ script. Three histological processing of samples, including von Kossa silver nitrate, toluidine blue, and fluorescence by the deposition of calcein were utilized.
Results
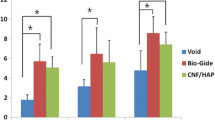
Zn-membranes (Zn-Ms) promoted the highest amount of new bone and higher bone perimeter than both unloaded and Ti-membranes (Ti-Ms). Ca-membranes (Ca-Ms) attained higher osteoid perimeter and bone perimeter than Zn-Ms. The skeleton analysis showed that Zn-Ms produced more branches and junctions at the trabecular bone than BMP-loaded membranes (BMP-Ms). Samples treated with Ti-Ms showed less bone formation and bony bridging processes. Both Zn-Ms and Ca-Ms achieved higher number of osteoblasts than the control group. BMP-Ms and Ca-Ms originated higher number of blood vessels than Ti-Ms and control group.
Conclusions
Zn incorporation in novel nanostructured membranes provided the highest regenerative efficiency for bone healing at the rabbit calvarial defects.
Clinical relevance
Zn-Ms promoted osteogenesis and enhanced biological activity, as mineralized and osteoid new bone with multiple interconnected ossified trabeculae appeared in close contact with the membrane.






Similar content being viewed by others
References
Zhang F, Zhang WB, Shi Z, Wang D, Jin J, Jiang L (2013) Nanowire-haired inorganic membranes with superhydrophilicity and underwater ultralow adhesive superoleophobicity for high-efficiency oil/water separation. Adv Mater Deerfield Beach Fla 25:4192–4198. https://doi.org/10.1002/adma.201301480
Omar O, Elgali I, Dahlin C, Thomsen P (2019) Barrier membranes: more than the barrier effect? J Clin Periodontol 46:103–123. https://doi.org/10.1111/jcpe.13068
Sam G, Pillai BRM (2014) Evolution of barrier membranes in periodontal regeneration-“are the third generation membranes really here?”. J Clin Diagn Res JCDR 8:ZE14–ZE17. https://doi.org/10.7860/JCDR/2014/9957.5272
Punet X, Mauchauffé R, Rodríguez-Cabello JC, Alonso M, Engel E, Mateos-Timoneda MA (2015) Biomolecular functionalization for enhanced cell-material interactions of poly(methyl methacrylate) surfaces. Regen Biomater 2:167–175. https://doi.org/10.1093/rb/rbv014
Kim S, Hwang Y, Kashif M, Jeong D, Kim G (2016) Evaluation of bone regeneration on polyhydroxyethyl-polymethyl methacrylate membrane in a rabbit calvarial defect model. Vivo Athens Greece 30:587–591
Osorio R, Alfonso-Rodríguez CA, Osorio E, Medina-Castillo AL, Alaminos M, Toledano-Osorio M, Toledano M (2017) Novel potential scaffold for periodontal tissue engineering. Clin Oral Investig 21:2695–2707. https://doi.org/10.1007/s00784-017-2072-8
Castillo-Dalí G, Castillo-Oyagüe R, Terriza A, Saffar JL, Batista-Cruzado A, Lynch CD, Sloan AJ, Gutiérrez-Pérez JL, Torres-Lagares D (2016) Pre-prosthetic use of poly(lactic-co-glycolic acid) membranes treated with oxygen plasma and TiO2 nanocomposite particles for guided bone regeneration processes. J Dent 47:71–79. https://doi.org/10.1016/j.jdent.2016.01.015
Nandakumar A, Yang L, Habibovic P, van Blitterswijk C (2010) Calcium phosphate coated electrospun fiber matrices as scaffolds for bone tissue engineering. Langmuir ACS J Surf Colloids 26:7380–7387. https://doi.org/10.1021/la904406b
Seol Y-J, Kim K-H, Kang YM, Kim IA, Rhee S-H (2009) Bioactivity, pre-osteoblastic cell responses, and osteoconductivity evaluations of the electrospun non-woven SiO2-CaO gel fabrics. J Biomed Mater Res B Appl Biomater 90:679–687. https://doi.org/10.1002/jbm.b.31334
Jovanovic SA, Hunt DR, Bernard GW, Spiekermann H, Wozney JM, Wikesjö UME (2007) Bone reconstruction following implantation of rhBMP-2 and guided bone regeneration in canine alveolar ridge defects. Clin Oral Implants Res 18:224–230. https://doi.org/10.1111/j.1600-0501.2006.01324.x
Saulacic N, Fujioka-Kobayashi M, Kobayashi E, Schaller B, Miron RJ (2017) Guided bone regeneration with recombinant human bone morphogenetic protein 9 loaded on either deproteinized bovine bone mineral or a collagen barrier membrane. Clin Implant Dent Relat Res 19:600–607. https://doi.org/10.1111/cid.12491
Sun Z, Kim JH, Zhao Y, Attard D, Dou SX (2013) Morphology-controllable 1D–3D nanostructured TiO2 bilayer photoanodes for dye-sensitized solar cells. Chem Commun 49:966–968. https://doi.org/10.1039/C2CC37212F
Udagawa A, Sato S, Hasuike A, Kishida M, Arai Y, Ito K (2013) Micro-CT observation of angiogenesis in bone regeneration. Clin Oral Implants Res 24:787–792. https://doi.org/10.1111/j.1600-0501.2012.02458.x
Erben RG, Jolette J, Chouinard L, Boyce R (2017) Application of histopathology and bone histomorphometry for understanding test article-related bone changes and assessing potential bone liabilities. In: Bone toxicology. Springer, Berlin, pp 253–278
Osorio R, Alfonso-Rodríguez CA, Medina-Castillo AL, Alaminos M, Toledano M (2016) Bioactive polymeric nanoparticles for periodontal therapy. PLoS One 11:e0166217. https://doi.org/10.1371/journal.pone.0166217
Suliman S, Xing Z, Wu X, Xue Y, Pedersen TO, Sun Y, Døskeland AP, Nickel J, Waag T, Lygre H, Finne-Wistrand A, Steinmüller-Nethl D, Krueger A, Mustafa K (2015) Release and bioactivity of bone morphogenetic protein-2 are affected by scaffold binding techniques in vitro and in vivo. J Control Release 197:148–157. https://doi.org/10.1016/j.jconrel.2014.11.003
Sánchez F, Orero A, Soriano A, Correcher C, Conde P, González A, Hernández L, Moliner L, Rodríguez-Alvarez MJ, Vidal LF, Benlloch JM, Chapman SE, Leevy WM (2013) ALBIRA: a small animal PET∕SPECT∕CT imaging system. Med Phys 40:051906. https://doi.org/10.1118/1.4800798
Doube M, Kłosowski MM, Arganda-Carreras I, Cordelières FP, Dougherty RP, Jackson JS, Schmid B, Hutchinson JR, Shefelbine SJ (2010) BoneJ: free and extensible bone image analysis in ImageJ. Bone 47:1076–1079. https://doi.org/10.1016/j.bone.2010.08.023
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Rubin MR, Zhou H, Cusano NE, Majeed R, Omeragic B, Gomez M, Nickolas TL, Dempster DW, Bilezikian JP (2018) The effects of long-term administration of rhPTH(1-84) in hypoparathyroidism by bone histomorphometry. J Bone Miner Res 33:1931–1939. https://doi.org/10.1002/jbmr.3543
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res Off J Am Soc Bone Miner Res 2:595–610. https://doi.org/10.1002/jbmr.5650020617
La Monaca G, Iezzi G, Cristalli MP, Pranno N, Sfasciotti GL, Vozza I (2018) Comparative histological and histomorphometric results of six biomaterials used in two-stage maxillary sinus augmentation model after 6-month healing. Biomed Res Int 2018(9430989):1–11. https://doi.org/10.1155/2018/9430989
Maggiano IS, Maggiano CM, Clement JG, Thomas CDL, Carter Y, Cooper DML (2016) Three-dimensional reconstruction of Haversian systems in human cortical bone using synchrotron radiation-based micro-CT: morphology and quantification of branching and transverse connections across age. J Anat 228:719–732. https://doi.org/10.1111/joa.12430
Chang Y-C, Ho K-N, Feng S-W, Huang H-M, Chang C-H, Lin C-T, Pan TN-C, YH CW-J (2016) Fibronectin-grafted titanium dental implants: an in vivo study. Biomed Res Int 2016:2414809. https://doi.org/10.1155/2016/2414809
Fujioka-Kobayashi M, Kobayashi E, Schaller B, Mottini M, Miron RJ, Saulacic N (2017) Effect of recombinant human bone morphogenic protein 9 (rhBMP9) loaded onto bone grafts versus barrier membranes on new bone formation in a rabbit calvarial defect model. J Biomed Mater Res A 105:2655–2661. https://doi.org/10.1002/jbm.a.36125
Augustine R, Malik HN, Singhal DK, Mukherjee A, Malakar D, Kalarikkal N, Thomas S (2014) Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J Polym Res 21:347. https://doi.org/10.1007/s10965-013-0347-6
Liu W, Li J, Cheng M, Wang Q, Yeung KWK, Chu PK, Zhang X (2018) Zinc-modified sulfonated polyetheretherketone surface with immunomodulatory function for guiding cell fate and bone regeneration. Adv Sci 5:1800749. https://doi.org/10.1002/advs.201800749
Dey A, Bomans PHH, Müller FA, Will J, Frederik PM, de With G, Sommerdijk NAJM (2010) The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat Mater 9:1010–1014. https://doi.org/10.1038/nmat2900
Guarnieri R, Belleggia F, DeVillier P, Testarelli L (2018) Histologic and histomorphometric analysis of bone regeneration with bovine grafting material after 24 months of healing. A case report. J Funct Biomater 9:E48. https://doi.org/10.3390/jfb9030048
Turri A, Dahlin C (2015) Comparative maxillary bone-defect healing by calcium-sulphate or deproteinized bovine bone particles and extra cellular matrix membranes in a guided bone regeneration setting: an experimental study in rabbits. Clin Oral Implants Res 26:501–506. https://doi.org/10.1111/clr.12425
Toledano M, Yamauti M, Ruiz-Requena ME, Osorio R (2012) A ZnO-doped adhesive reduced collagen degradation favouring dentine remineralization. J Dent 40:756–765. https://doi.org/10.1016/j.jdent.2012.05.007
Tayşi M, Atalay B, Çankaya B, Yıldırım S (2018) Effects of single- and double-layered resorbable membranes and platelet-rich fibrin on bone healing. Clin Oral Investig 22:1689–1695. https://doi.org/10.1007/s00784-017-2259-z
Schmitz JP, Schwartz Z, Hollinger JO, Boyan BD (1990) Characterization of rat calvarial nonunion defects. Acta Anat (Basel) 138:185–192
Ping Z, Wang Z, Shi J, Wang L, Guo X, Zhou W, Hu X, Wu X, Liu Y, Zhang W, Yang H, Xu Y, Gu Y, Geng D (2017) Inhibitory effects of melatonin on titanium particle-induced inflammatory bone resorption and osteoclastogenesis via suppression of NF-κB signaling. Acta Biomater 62:362–371. https://doi.org/10.1016/j.actbio.2017.08.046
Zhang N, Zhao D, Liu N, Wu Y, Yang J, Wang Y, Xie H, Ji Y, Zhou C, Zhuang J, Wang Y, Yan J (2018) Assessment of the degradation rates and effectiveness of different coated Mg-Zn-Ca alloy scaffolds for in vivo repair of critical-size bone defects. J Mater Sci Mater Med 29(138):138. https://doi.org/10.1007/s10856-018-6145-2
Oktay EO, Demiralp B, Demiralp B, Senel S, Cevdet Akman A, Eratalay K, Akincibay H (2010) Effects of platelet-rich plasma and chitosan combination on bone regeneration in experimental rabbit cranial defects. J Oral Implantol 36:175–184. https://doi.org/10.1563/AAID-JOI-D-09-00023
Park CK, Lee Y, Kim KH, Lee ZH, Joo M, Kim H-H (2014) Nrf2 is a novel regulator of bone acquisition. Bone 63:36–46. https://doi.org/10.1016/j.bone.2014.01.025
Mariani E, Lisignoli G, Borzì RM, Pulsatelli L (2019) Biomaterials: foreign bodies or tuners for the immune response? Int J Mol Sci 20:E636. https://doi.org/10.3390/ijms20030636
Pinese C, Lin J, Milbreta U, Li M, Wang Y, Leong KW, Chew SY (2018) Sustained delivery of siRNA/mesoporous silica nanoparticle complexes from nanofiber scaffolds for long-term gene silencing. Acta Biomater 76:164–177. https://doi.org/10.1016/j.actbio.2018.05.054
Yang Y, Wang K, Gu X, Leong KW (2017) Biophysical regulation of cell behavior—cross talk between substrate stiffness and nanotopography. Eng Beijing China 3:36–54. https://doi.org/10.1016/J.ENG.2017.01.014
Sadowska JM, Wei F, Guo J, Guillem-Marti J, Ginebra M-P, Xiao Y (2018) Effect of nano-structural properties of biomimetic hydroxyapatite on osteoimmunomodulation. Biomaterials 181:318–332. https://doi.org/10.1016/j.biomaterials.2018.07.058
Acknowledgments
The authors thank the technical support of Álvaro Carrasco-Carmona for the manuscript edition.
Data availability statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Funding
Project MAT2017-85999-P MINECO/AEI/FEDER/UE supported by the Ministry of Economy and Competitiveness (MINECO) and European Regional Development Fund (FEDER).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The experiment was developed in accordance with the guidelines of the US National Institute of Health (NIH for Care and Use of Laboratory Animals) and European Directive 86/609/EEC regarding the care and use of animals for experimentation. The study also complied with the European Directive 2010/63/EU about the protection of animals used for scientific purposes and with all local laws and regulations. Animals were adequately housed; food and water were provided daily ad libitum with rabbit-maintenance Harlan-Teckland Lab Animal Diets (2030). The researchers obtained the approval of the Ethics Committee of the Institution (CCMI-Ref 028/16).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 438 kb)
Rights and permissions
About this article
Cite this article
Toledano, M., Gutierrez-Pérez, J.L., Gutierrez-Corrales, A. et al. Novel non-resorbable polymeric-nanostructured scaffolds for guided bone regeneration. Clin Oral Invest 24, 2037–2049 (2020). https://doi.org/10.1007/s00784-019-03068-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03068-8