Abstract
Objective
To evaluate the hard tissue volumetric and soft tissue contour linear changes in implants with two different implant surface characteristics after a ligature-induced peri-implantitis.
Material and methods
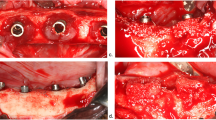
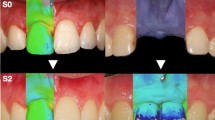
In eight beagle dogs, implants with the same size and diameter but distinct surface characteristics were placed in the healed mandibular sites. Test implants had an external monolayer of multi-phosphonate molecules (B+), while control implants were identical but without the phosphonate-rich surface. Once the implants were osseointegrated, oral hygiene was interrupted and peri-implantitis was induced by placing subgingival ligatures. After 16 weeks, the ligatures were removed and peri-implantitis progressed spontaneously. Bone to implant contact (BIC) and bone loss (BL) were assessed three-dimensionally with Micro-Ct (μCT). Dental casts were optically scanned and the obtained digitalized standard tessellation language (STL) images were used to assess the soft tissue vertical and horizontal contour linear changes.
Results
Reduction of the three-dimensional BIC percentage during the induction and progression phases of the experimental peri-implantitis was similar for both the experimental and control implants, without statistically significant differences between them. Soft tissue analysis revealed for both implant groups an increase in horizontal dimension after the induction of peri-implantitis, followed by a decrease after the spontaneous progression period. In the vertical dimension, a soft tissue dehiscence was observed in both groups, being more pronounced at the buccal aspect.
Conclusions
The added phosphonate-rich surface did not provide a more resistant environment against experimental peri-implantitis, when assessed by the changes in bone volume and soft tissue contours.
Clinical relevance
Ligature-induced peri-implantitis is a validated model to study the tissue changes occurring during peri-implantitis. It was hypothesized that a stronger osseointegration mediated by the chemical bond of a phosphonate-rich implant surface would develop an environment more resistant to the inflammatory changes occurring after experimental peri-implantitis. These results, however, indicate that the hard and soft tissue destructive changes occurring at both the induction and progression phases of experimental peri-implantitis were not influenced by the quality of osseointegration.








Similar content being viewed by others
References
Moraschini V, da C Poubel LA, Ferreira VF, dos S P Barboza E (2015) Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: a systematic review. Int J Oral Maxillofac Surg 44:377–388
Pjetursson BE, Asgeirsson AG, Zwahlen M, Salier I (2014) Improvements in implant dentistry over the last decade: comparison of survival and complication rates in older and newer publications. Int J Oral Maxillofac Implants 29(suppl):308–324
Caton J, Armitage G, Berglundh T et al (2018) A new classification scheme for periodontal and peri- implant diseases and conditions – introduction and key changes from the 1999 classification. J Clin Periodontol 45(Suppl 20):S1–S8
Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, Chen S, Cochran D, Derks J, Figuero E, Hämmerle CHF, Heitz-Mayfield LJA, Huynh-Ba G, Iacono V, Koo KT, Lambert F, McCauley L, Quirynen M, Renvert S, Salvi GE, Schwarz F, Tarnow D, Tomasi C, Wang HL, Zitzmann N (2018) Peri- implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol 45(Suppl 20):S286–S291
Schwarz F, Derks J, Monje A, Wang HL (2018) Peri-implantitis. J Clin Periodontol 45(Suppl 20):S246–S266
Derks J, Tomasi C (2015) Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol 42(Suppl. 16):S158–S171
Krebs M, Kesar N, Begic A, von Krockow N, Nentwig G-H, Weigl P (2019) Incidence and prevalence of peri-implantitis and peri-implant mucositis 17 to 23 (18.9) years postimplant placement. Clin Implant Dent Relat Res 21:1116–1123. https://doi.org/10.1111/cid.12848
Renvert S, Persson GR, Pirih FQ, Camargo PM (2018) Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Clin Periodontol 45(Suppl 20):S278–S285
Ivanosky S, Lee R (2017) Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontol 0:1–15
Giovannoli JL, Roccuzzo M, Albouy JP, Duffau F, Lin GH, Serino G (2019) Local risk indicators - consensus report of working group 2. Intern Dent J 69(Suppl):7–11
Dreyer H, Grischke J, Tiede C et al (2018) Epidemiology and risk factors of peri-implantitis: a systematic review. J Periodontal Res 00:1–25
Saulaci N, Schaller B (2019) Prevalence of peri-implantitis in implants with turned and rough surfaces: a systematic review. J Oral Maxillofac Res 10(e1):1–12
Jordana F, Susbielles L, Colat-Parros J (2018) Periimplantitis and implant body roughness: a systematic review of literature. Implant Dent 27(6):672–681
Asensio G, Vazquez-Lasa B, Rojo L (2019) Achievements in the topographic design of commercial titanium dental implants: towards anti-peri-implantitis surfaces. J Clin Med 8(11):1982–2000
Jansen JA, Brugge P, Van Der Waal E, Vredenberg A, Wolke J (2003) Osteocapacities of calcium phosphate ceramics. In: Ellingsen JE, Lyngstadaas SP (eds) Bioimplant inter- face. CRC, Boca Raton, pp 305–322
Junker R, Dimakis A, Thoneick M, Jansen JA (2009) Effects of implant surface coatings and composition on bone integration: a systematic review. Clin Oral Implants Res 20(Suppl. 4):185–206
Viornery C, Chevolot Y, Léonard D, Aronsson BO, Péchy P, Mathieu HJ, Descouts P, Grätzel M (2002) Surface modification of titanium with phosphonic acid to improve bone bonding: characterization by XPS and ToF-SIMS. Langmuir 18:2582–2589
Viornery C, Guenther HL, Aronsson BO, Péchy P, Descouts P, Grätzel M (2002) Osteoblast culture on polished titanium disks modified with phosphonic acids. J Biomed Mater Res 62:149–155
Von Salis-Soglio M, Stübinger S, Sidler M, Klein K, Ferguson S, Kämpf K et al (2014) A novel multi-phosphonate surface treatment of titanium dental implants: a study in sheep. J Funct Biomater 5(3):135–157
Esposito M, Dojcinovic I, Germon L, Lévy N, Curno R, Buchini S, Péchy P, Aronsson BO (2013) Safety and efficacy of a biomimetic monolayer of permanently bound multi-phosphonic acid molecules on dental implants: 1year post-loading results from a pilot quadruple-blinded randomised controlled trial. Eur J Oral Implantol 6:227–236
Vignoletti F, Abrahamsson I (2012) Quality of reporting of experimental research in implant dentistry. Critical aspects in design, outcome assessment and model validation. J Clin Periodontol 39(Suppl. 12):6–27
Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C (1992) Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res 3(1):9–16
Feldkamp LA, Davis LC, Kress JW (1984) Practical cone-beam algorithm. J Opt Soc Am 1(6):612–619
Becker K, Wilmes B, Grandjean C, Drescher D (2018) Impact of manual control point selection accuracy on automated surface matching of digital dental models. Clin Oral Investig 22(2):801–810
Di Raimondo R, Sanz-Esporrin J, Pla R, Sanz-Martin I, Luengo F, Vignoletti F, Nuñez J, Sanz M (2020) Alveolar crests contour changes after guided bone regeneration using different biomaterials: an experimental in vivo investigation. Clin Oral Investig 24(7):2351–2361
Di Raimondo R, Sanz-Esporrin J, Sanz-Martin I, Pla R, Luengo F, Vignoletti F, Nuñez J, Sanz M (2020) Hard and soft tissue changes after guided bone regeneration using two different barrier membranes: an experimental in vivo investigation. Clin Oral Investig. https://doi.org/10.1007/s00784-020-03537-5
Godoy-Gallardo M, Manzanares-Cespedes MC, Sevilla P, Nart J, Manzanares N, Manera JM, Gil FJ, Body SK, Rodriguez D (2016) Evaluation of bone loss in antibacterial coated dental implants: an experimental study in dogs. Mater Sci Eng C Mater Biol Appl 69:538–545
Berglundh T, Gotfredsen K, Zitzmann NU, Lang NP, Lindhe J (2007) Spontaneous progression of ligature induced peri-implantitis at implants with different surface roughness: an experimental study in dogs. Clin Oral Implants Res 18:655–661
Albouy JP, Abrahamsson I, Persson LG, Berglundh T (2008) Spontaneous progression of peri-implantitis at different types of implants. An experimental study in dogs. I: clinical and radiographic observations. Clin Oral Implants Res 19(10):997–1002
Albouy JP, Abrahamsson I, Persson LG, Berglundh T (2009) Spontaneous progression of ligatured induced peri-implantitis at implants with different surface characteristics. An experimental study in dogs II: histological observations. Clin Oral Implants Res 20(4):366–371
Fickl S, Kebschull M, Calvo-Guirado JL, Hurzeler M, Zuhr O (2015) Experimental peri-implantitis around different types of implants – a clinical and radiographic study in dogs. Clin Implant Dent Relat Res 17(Suppl 2):e661–e669
Sanz-Esporrin J, Blanco J, Sanz-Casado JV, Muñoz F, Sanz M (2019) The adjunctive effect of rhBMP-2 on the regeneration of peri-implant bone defects after experimental peri-implantitis. Clin Oral Implants Res 30(12):1209–1219
Maglione M, Vaccari L, Mancini L, Ciancio R, Bedolla DE, Bevilacqua L, Tonellato P (2019) Micro-ATR FTIR, SEM-EDS and X-ray micro-CT: an innovative multi-technique approach to investigate bone affected by peri-implantitis. Int J Oral Maxillofac Implants 34(3):631–641
Finelle G, Papadimitriou DEV, Souza AB, Katebi N, Gallucci GO, Araujo MG (2015) Peri-implant soft tissue and marginal bone adaptation on implant with non-matching healing abutments: micro-CT analysis. Clin Oral Implants Res 26:e42–e46
Thoma DS, Jung U-W, Park J-Y, Bienz SP, Husler J, Jung RE (2017) Bone augmentation at peri-implant dehiscence defects comparing a synthetic polyethylene glycol hydrogel matrix versus standard guided bone regeneration techniques. Clin Oral Implants Res 28:e76–e83
Khobragade P, Jain A, Setlur Nagesh SV, Andreana S, Dziak R, Sunkara SK, Ionita CN (2015) Micro-computed tomography (CT) based assessment of dental regenerative therapy in the canine mandible model. Proc SPIE Int Soc Opt Eng 17:9417
Qian W, Qiu J, Liu X (2019) Minocycline hydrochloride loaded graphene oxide films on implant abutments for peri-implantitis treatment in beagle dogs. J Periodontol 91:792–799. https://doi.org/10.1002/JPER.19-0285
Sanz-Martin I, Benic GI, Hammerle CH, Thoma DS (2016) Prospective randomized controlled clinical study comparing two dental implant types: volumetric soft tissue changes at 1 year of loading. Clin Oral Implants Res 27:406–411
Sanz-Martin I, Vignoletti F, Nuñez J, Permuy M, Muñoz F, Sanz-Esporrin J, Fierravanti L, Shapira L, Sanz M (2017) Hard and soft tissue integration of immediate and delayed implants with a modified coronal macro design: histological, micro CT and volumetric soft tissue changes from a pre-clinical in vivo study. J Clin Periodontol 44(8):842–853
Sanz-Martin I, Ferrantino L, Vignoletti F, Nunez J, Baldini N, Duvina M, Alcaraz J, Sanz M (2018) Contour changes after guided bone regeneration of large non-contained mandibular buccal bone defects using deproteinized bovine bone mineral and a porcine-derived collagen membrane: an experimental in vivo investigation. Clin Oral Investig 22(3):1273–1283
Basler T, Naenni N, Schneider D, Hammerle CHF, Jung R, Thoma DS (2018) Randomized controlled clinical study assessing two membranes for guided bone regeneration of peri-implant bone defects: 3-year results. Clin Oral Implants Res 29(5):499–507
Galarraga-Vinueza ME, Obreja K, Magini R, Sculean A, Sader R, Schwarz F (2020) Volumetric assessment of tissue changes following combined surgical therapy of peri-implantitis: a pilot study. J Clin Periodontol 00:1–10. https://doi.org/10.1111/jcpe.13335
Mehl A, Gloger W, Kunzelmann KH, Hickel R (1997) A new optical 3-D device for the detection of wear. J Dent Res 76(11):1799–1807
Windisch SI, Jung RE, Sailer I, Studer SP, Ender A, Hämmerle CHF (2007) A new optical method to evaluate three-dimensional volume changes of alveolar contours: a methodological in vitro study. Clin Oral Implants Res 18:545–551
Acknowledgments
The authors would like to express their gratitude to Liebert Parreiras Nogueira at the Clinical Oral Research Laboratory, Faculty of Dentistry, University of Oslo for their help preparing and analyzing the microCT data.
Funding
This study was partially supported with a research contract between the University Complutense of Madrid and MIS Dental Implants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
AE-LU-001/04/16.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary graph 1
Graphic representation of the hard tissue volumetric analysis obtained by means of μCT. Blue and green rectangles corresponded to test and control group, respectively. (PNG 187 kb)
Rights and permissions
About this article
Cite this article
Di Raimondo, R., Sanz-Esporrin, J., Martin, I.S. et al. Hard tissue volumetric and soft tissue contour linear changes at implants with different surface characteristics after experimentally induced peri-implantitis: an experimental in vivo investigation. Clin Oral Invest 25, 3905–3918 (2021). https://doi.org/10.1007/s00784-020-03720-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03720-8